
 Fe��OH��3+3H+
Fe��OH��3+3H+ Fe��OH��3+3H+
Fe��OH��3+3H+
| ||
| ||
 Fe��OH��3+3H+��
Fe��OH��3+3H+�� Fe��OH��3+3H+��
Fe��OH��3+3H+��
| ||
| ||


 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013������ʡ��������УЭ��������һ�����Ͽ��Ի�ѧ�Ծ����������� ���ͣ������
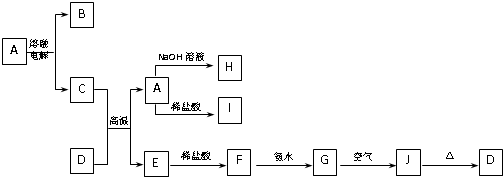
�� 7��) A��J����ѧ��ѧ���������ʣ�����֮���ת����ϵ����ͼ��ʾ�����ֲ�������ȥ������֪A��һ�ָ��۵����ʣ�D��һ�ֺ���ɫ���塣
��ش��������⣺
��1������A�ľ�������Ϊ___________��K�Ļ�ѧʽΪ ��
��2��C��D�ڸ����µķ�Ӧ��ұ��ҵ�ϳ�Ϊ ��Ӧ��
��3��G��J�Ļ�ѧ����ʽΪ ��
��4��A��H�����ӷ���ʽΪ ��
��5��I��ˮ��ҺpH_____7���>������<����=������ԭ���� �������ӷ���ʽ��ʾ����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com