
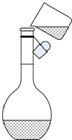
 ʵ��������Ҫ����500mL0.10mol/L��NaOH��Һ���ش��������⣮
ʵ��������Ҫ����500mL0.10mol/L��NaOH��Һ���ش��������⣮| n |
| V |
| n |
| V |
| 10mL |
| 20mL |
| n |
| V |
| n |
| V |
| n |
| V |


 ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
ȫ�ŵ�����Ԫ�ƻ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Al3+��Cu2+��SO42-��Cl- |
| B��NH4+��Mg2+��SO42-��Cl- |
| C��Na+��HCO3-��K+��OH- |
| D��Mg2+��K+��Cl-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ͬѧ��Ϊ��̽��þ�������ᡢ���ᷴӦʱ��Ũ�Ȼ��¶ȶԷ�Ӧ���ʣ��۲�þ����ʧ��ʱ�䣩��Ӱ�죬�����������»�ѧ��Ʒ��0.2mol/L��0.40mol/L��HCl��Һ��0.2mol/L��0.40mol/L��CH3COOH��Һ��4��þ������״����С��������ͬ������֧�Թܺͽ�ͷ�ιܣ���Һ�¶ȿ���Ϊ298K��308K��
ͬѧ��Ϊ��̽��þ�������ᡢ���ᷴӦʱ��Ũ�Ȼ��¶ȶԷ�Ӧ���ʣ��۲�þ����ʧ��ʱ�䣩��Ӱ�죬�����������»�ѧ��Ʒ��0.2mol/L��0.40mol/L��HCl��Һ��0.2mol/L��0.40mol/L��CH3COOH��Һ��4��þ������״����С��������ͬ������֧�Թܺͽ�ͷ�ιܣ���Һ�¶ȿ���Ϊ298K��308K��| ʵ�� ��� |
�¶ȣ�K�� | ����Ũ�� ��mol?L-1�� |
����Ũ�ȣ�mol?L-1�� | ʵ��Ŀ�� |
| �� | 298 | 0.20 | / | a��ʵ��ٺ͢���̽�� b��ʵ��ٺ͢���̽�� c��ʵ��ٺ͢���̽����ͬ�¶��£���ͬŨ�ȵ����ᡢ������þ��Ӧ���ʵ����� |
| �� | 308 | 0.20 | / | |
| �� | 298 | 0.40 | / | |
| �� | 0.20 | / |
| 1 |
| 2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| Ӧ����NaOH������/g | �Ѹ����� | ���Ѹ��������Ҫ�IJ������� |
| �� | �ձ�����Ͳ����ͷ�ιܡ�500mL����ƿ | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��Cl2��ˮ��Һ���Ե��磬˵��Cl2�ǵ���� |
| B���ڸ��������£�C���û���SiO2�е�Si��˵��C��������ǿ��Si |
| C������۵⻯�ص���Һ�м�����ˮ����Һ��Ϊ��ɫ��˵��Cl2��������ǿ��I2 |
| D���ڵ��з�̪��Na2CO3��Һ�У�����BaCl2��Һ����Һ��ɫ��˵��BaCl2��Һ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��a% |
| B��2a% |
| C��1-1.75a% |
| D��1-0.75a% |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��4 g��ˮ��D2O��������������Ϊ0.2NA |
| B��4.48 L H2��O2�Ļ������������������Ϊ0.2NA |
| C��0.2 mol Cl2�ܽ��ڵ������ˮ�У�ת�Ƶ�����Ϊ0.2NA |
D��12.6 g�����谷���ṹ����ͼ������̼����������ĿΪ0.6NA |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com