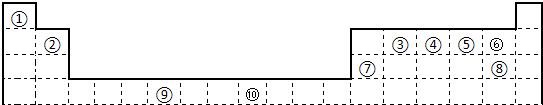
�±�Ϊ��ʽ���ڱ���һ���֣����еı�Ŵ�����Ӧ��Ԫ�أ�

��ش��������⣺
��1��ͼ1������d����Ԫ����
��
��
�����ţ���
��2����ѧ���֣��ڡ��ܡ�������Ԫ�ص�ԭ���γɵľ�����г����ԣ��侧���Ľṹ�ص���ͼ2��ʾ��ͼ�Тڡ��ܡ���ֱ�λ��
���������ġ����㡢���ģ�����û�����Ļ�ѧʽΪ
MgCNi3��CMgNi3
MgCNi3��CMgNi3
���ö�Ӧ��Ԫ�ط��ű�ʾ����
��3��Ԫ�آڵ�һ���⻯������Ҫ�Ļ���ԭ�ϣ����Ѹ��⻯��IJ�����Ϊ����ʯ�ͻ�����չˮƽ�ı�־���йظ��⻯����ӵ�˵����ȷ����
BD
BD
��
A�������к��з��Ӽ����� B�����ں��м��Լ��ķǼ��Է���
C��ֻ����4��sp-s�ĦҼ���1��p-p�Ħм�D�����⻯������Т�ԭ�Ӳ���sp
2�ӻ�
��4��ijԪ�صļ۵����Ų�ʽΪns
nnp
n+1����Ԫ��ԭ�ӵĺ����������ӵŶԵ�����Ϊ
1
1
����Ԫ����Ԫ�آ��γɵ�18���ӵ�X���ӣ�������32���ĵ���ʽΪ
����Ԫ�ػ�����Ԫ�آ��γ�10���ӵ��������Y��������17������������Y����ͨ��ʢ������ͭˮ��Һ���Թ������Ϊ
�Ȳ�����ɫ�������������ʧ���������ɫ����Һ
�Ȳ�����ɫ�������������ʧ���������ɫ����Һ
��
��5���±�Ϊԭ��������������Ķ�����Ԫ��A��F�ĵ�һ��������������ݣ�
| ������I��eV�� |
A |
B |
C |
D |
E |
F |
| I1 |
11.3 |
14.5 |
13.6 |
5.2 |
7.6 |
6.0 |
| I2 |
24.4 |
29.6 |
35.1 |
49.3 |
15.0 |
18.8 |
| I3 |
47.9 |
47.4 |
54.9 |
71.6 |
80.1 |
28.4 |
| I4 |
64.5 |
77.5 |
77.4 |
98.9 |
109.2 |
112.0 |
| I5 |
392.1 |
97.9 |
113.9 |
138.3 |
141.3 |
153.7 |
��ش𣺱��еĽ���Ԫ����
DEF
DEF
������ĸ������A��B��CΪԭ���������������ͬ��������Ԫ�أ�������ʾB��A��C�ĵ�һ�����ܶ��Դ���ԭ����
B��np����ϵĵ��Ӱ�����������A��C�ͣ��������ȶ������Ե�һ�����ܱ�A��C��
B��np����ϵĵ��Ӱ�����������A��C�ͣ��������ȶ������Ե�һ�����ܱ�A��C��
��

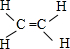 ��Ϊƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�������ʯ���ѽ����ɣ�
��Ϊƽ���ͽṹ������Ϊ�Գƽṹ�����ڷǽ����Է��ӣ�������ʯ���ѽ����ɣ�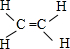 �������к���5���Ҽ���1���м�����C����
�������к���5���Ҽ���1���м�����C����

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�