
X��Y��Z��W��T��Ԫ�����ڱ���ǰ�����ڵ�����Ԫ�ء��й�������Ԫ�ص���Ϣ���±���
| Ԫ�ش��� | �����Ϣ |
| X | ����һ�ֺ��ص�ԭ�Ӻ���û������ |
| Y | ����ɺܶ������ʲ�ͬ�ĵ��ʣ�Ҳ���γɻ�������������һ��Ԫ�� |
| Z | ��̬ԭ�ӵ����������Ų�ʽΪnsnnpn+1 |
| W | ����Ԫ����������ֻ������Щ��������ˮ��Ӧ�����ɼ����ƽ������W3����Ϊ�����������ɡ�� |
| T | ��Ҫ���ϼ���+1��+2�ۣ������������ˮ��Һ�г���ɫ |
 YZ- (aq)+X3W+(aq) ��H="+45.6" KJ/mol
YZ- (aq)+X3W+(aq) ��H="+45.6" KJ/mol��1���ڶ����ڵڢ�A �� 1S2 2S2 2P6 3S2 3P6 3d104s1
��2��O N ��O ��C
��3��
��4��CH4+4CuO  4Cu+CO2+2H2O
4Cu+CO2+2H2O
��5��HCN(aq)+OH-(aq)=CN-(aq)+H2O��1�� ��H="-11.7" kJ/mol
�������������������Ŀ�ṩ����Ϣ��֪��X��H��Y��C��Z��N��W��O��T��Cu����1��OԪ�������ڱ��е�λ����λ�ڵڶ����ڵڢ�A��29��Ԫ����Cu������ԭ�Ӻ���ĵ����ڸ���������Ų����ڰ������ȫ������ȫ��ʱ���ȶ���״̬������Cu�Ļ�̬�����Ų�ʽ1S2 2S2 2P6 3S2 3P6 3d104s1����2��Ԫ�صķǽ�����Խǿ����縺�������������Ԫ���зǽ�������ǿ����OԪ�ء����Ե縺��������O��һ������£�ͬһ���ڵ�Ԫ�أ�Ԫ�ص�ԭ�Ӱ뾶ԽС��Ԫ��ʧȥ���Ӿ�Խ�ѣ������ܾ�Խ����ԭ�Ӻ���ĵ����ڸ���������Ų����ڰ������ȫ������ȫ��ʱ���ȶ���״̬��C��N��O����Ԫ�صĵ�һ������˳��ΪN ��O ��C����3�����ӻ�����NH5�ĵ���ʽΪ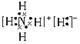 ����4�����������£�TW��Y����⻯�ﷴӦ�Ļ�ѧ����ʽ��CH4+4CuO
����4�����������£�TW��Y����⻯�ﷴӦ�Ļ�ѧ����ʽ��CH4+4CuO  4Cu+CO2+2H2O����5�����٣��ڣ������ɵ�HCN(aq)+OH-(aq) =CN-(aq)+H2O��1�� ��H="-11.7" kJ/mol��
4Cu+CO2+2H2O����5�����٣��ڣ������ɵ�HCN(aq)+OH-(aq) =CN-(aq)+H2O��1�� ��H="-11.7" kJ/mol��
���㣺����Ԫ�ص��ƶϡ�ԭ�ӵĵ����Ų�ʽ��Ԫ�ص�λ�á�����ʽ����д���縺�ԵıȽϡ���ѧ����ʽ���Ȼ�ѧ����ʽ����д��֪ʶ��


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��������9������ ��
�� ��
�� ��
��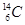 ��
��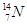 ��
��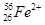 ��
��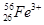 ��
��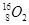 ��
��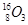 ����Ҫ��������¸��⣺
����Ҫ��������¸��⣺
(1) 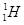 ��
��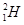 ��
��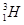 �ֱ�����Ԫ�ص�һ�� �����ǻ���Ϊ ��
�ֱ�����Ԫ�ص�һ�� �����ǻ���Ϊ ��
(2)��Ϊͬ������������� ��
(3)���� ��������Ҫ�����һ��(Ҫ��ÿС��ֱ�д������������������ӣ��Ҵ�������������ѡ��)��
��������Ҫ�����һ��(Ҫ��ÿС��ֱ�д������������������ӣ��Ҵ�������������ѡ��)��
��Z��n��b��ͬ��A��ͬ�� ��
��A��n��b��ͬ��Z��ͬ�� ��
��A��Z��b��ͬ��n��ͬ�� ��
��Z��n��A��ͬ��b��ͬ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ԫ�����ڱ���ѧϰ��ѧ����Ҫ���ߣ�������������Ϣ���ɡ��±����������ֶ�����Ԫ�ص�ԭ�Ӱ뾶����Ҫ���ϼۣ�
| Ԫ�ش��� | A | B | C | D | E |
| ԭ�Ӱ뾶/nm | 0.16 | 0.143 | 0.102 | 0.089 | 0.074 |
| ��Ҫ���ϼ� | ��2 | ��3 | ��6����2 | ��2 | ��2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��.ijԪ����6�����Ӵ��ڵ������Ӳ�d����ϣ��Ʋ��Ԫ�ص�ԭ��������________�������ݺ��ع����Ʋ��ڵ������Ӳ�d�����δ�ɶԵ��ӵĸ�����________����
��.�˵����С��18������Ԫ�أ�Aԭ������������Ϊa��������������Ϊb����Bԭ��M�������Ϊ��a��b������L��Ϊ��a��b��������
��1��д��Ԫ�ص����ƺͷ��ţ�A��____________________��B.____________________��
��2��д����ԭ�ӵĺ�������Ų��Ĺ����ʾʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ֶ�����Ԫ�ص����ʻ�ṹ��Ϣ���±����������Ϣ�ش��������⡣
| Ԫ�� | A | B | C | D |
| ���ʽṹ��Ϣ | ԭ��������������Ϊ���Ӳ�����2����������Ϊ����ɫ���壬��ȼ����ΪQ KJ/mol | ���ʳ��¡���ѹ�������壬������ˮ��ԭ�ӵ�M��p�����1��δ�ɶԵĵ��� | ��������������ɫ���塢������ǿ�������ڿ�����ȼ�շ�����ɫ�Ļ��档 | ԭ���������Ӳ���s����������p��������������һ����Ҫ�İ뵼����ϡ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
Ԫ�������ڱ��е�λ�ã���ӳ��Ԫ�ص�ԭ�ӽṹ��Ԫ�ص����ʡ���ͼ��Ԫ�����ڱ���һ���֡�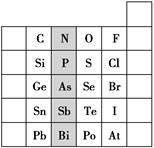
(1)��Ӱ����Ԫ����Ԫ�����ڱ��е�λ��Ϊ________�塣
(2)����Ԫ��������Ԥ�⣺H3AsO4����ǿ�ỹ�����________��
(3)C��SiԪ�ص��⻯�ﶼ����ȼ�գ���SiԪ�ص��⻯���ڿ����п�����ȼ����ԭ����_________________________________________��
��д��Si���⻯����ȫȼ�յĻ�ѧ����ʽ��______________________________��
(4)O2��H2�ķ�Ӧ�Dz����淴Ӧ����S��H2��Ӧ��һ���ȣ���д��Se��H2��Ӧ�Ļ�ѧ����ʽ��______________________
(5)�ԱȽ�S��O��F����Ԫ�ص�ԭ�Ӱ뾶��С��________(��Ԫ�ط���)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��C��D���Ƕ�����Ԫ�أ�ԭ�Ӱ뾶D>C>A>B������A��B����ͬһ���ڣ�A��C����ͬһ���塣Cԭ�Ӻ�������������A��Bԭ�Ӻ���������֮�ͣ�Cԭ��������ϵĵ�������Dԭ��������������4�����Իش�
(1)������Ԫ�طֱ��ǣ�A________��B________��C________��D________��
(2)������Ԫ�����ڳ��³�ѹ�µ�Һ̬����̬�⻯����ȶ����ɴ��С��˳����________��
(3)A��B�γɵ���ԭ�ӷ��ӵĵ���ʽ��________��B��D�γɵ�ԭ�Ӹ�����Ϊ1��1�Ļ�����ĵ���ʽ��________��
(4)AԪ��ij��������DԪ��ij�����ﷴӦ���ɵ��ʵĻ�ѧ����ʽ��____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��
| A | | C |
| | B | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
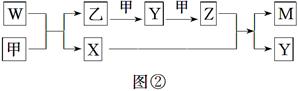
A��B��D��E����Ԫ�ؾ�Ϊ������Ԫ�أ�ԭ������������AԪ��ԭ�ӵĺ�������������Ӳ�������������������ȡ�B��D��E����Ԫ�������ڱ��е����λ����ͼ����ʾ��ֻ��EԪ�صĵ�������ˮ��Ӧ���������ᡣ�ס��ҡ�M��W��X��Y��Z�������ʾ���A��B��D����Ԫ���е�һ�ֻ�����ɣ�����ֻ��M����ͬʱ��������Ԫ�أ�WΪA��B��Ԫ����ɵ�18���ӷ��ӣ��������ȼ�ϣ��ס���Ϊ�ǽ������ʣ�X���Ӻ���10�����ӡ�����֮���ת����ϵ��ͼ����ʾ��
| B | D | |
| | | E |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com