
��1����֪���ױȰ����ȶ�����֪��4P(���ף�s��+5O2��g����2P2O5��s�� ��H1��
4P�����ף�s��+5O2��g����2P2O5��s�� ��H2����H1�ͦ�H2�Ĺ�ϵ�ǡ�H1 ��H2���������������
��������
��2����֪H2��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-726.5kJ��mol-1��д����CO2
��H2����Һ̬�״���Һ̬ˮ���Ȼ�ѧ����ʽ ��
��3����֪һ���¶��£����з�Ӧ��ƽ�ⳣ����SO2(g)+1/2O2(g) 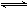 SO3(g)
K1,CO(g)+1/2O2(g)
SO3(g)
K1,CO(g)+1/2O2(g)
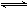 CO2(g)
K2������ͬ�¶��·�ӦSO2(g)+CO2(g)
CO2(g)
K2������ͬ�¶��·�ӦSO2(g)+CO2(g) 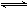 SO3(g)+CO(g)��ƽ�ⳣ��Ϊ
��
SO3(g)+CO(g)��ƽ�ⳣ��Ϊ
��
����K1��K2��ʾ��
����6�֣��� ��2��3H2(g)��CO2(g)��CH3OH��l��H2O(l)
��H����130.9 kJ/mol ��3��
��������
�����������1�����ױȰ����ȶ�����˵���������������ڰ�����������������ת��Ϊ���������ȷ�Ӧ����H��0�����ݷ�Ӧ��4P(���ף�s��+5O2��g����2P2O5��s�� ��H1�͢�4P�����ף�s��+5O2��g����2P2O5��s�� ��H2�����ݸ�˹���ɿ�֪���ڣ��ټ��õ���Ӧ4P�����ף�s����4P(���ף�s������÷�Ӧ�ġ�H����H2����H1��0�����ԡ�H1����H2��
��2��H2��g����CH3OH��l����ȼ���ȡ�H�ֱ�Ϊ-285.8kJ��mol-1��-726.5kJ��mol-1�����з�Ӧ��H2(g)�� O2(g)��H2O(l) ��H����285.8kJ/mol����CH3OH��l����
O2(g)��H2O(l) ��H����285.8kJ/mol����CH3OH��l���� O2(g)��2H2O(l)��CO2(g)
��H����726.5kJ/mol������ݸ�˹���ɿ�֪���١�3���ڼ��õ���Ӧ3H2(g)��CO2(g)��CH3OH��l��H2O(l)����˸÷�Ӧ�ķ�Ӧ�ȡ�H����285.8kJ/mol��3��726.5kJ/mol����130.9 kJ/mol��
O2(g)��2H2O(l)��CO2(g)
��H����726.5kJ/mol������ݸ�˹���ɿ�֪���١�3���ڼ��õ���Ӧ3H2(g)��CO2(g)��CH3OH��l��H2O(l)����˸÷�Ӧ�ķ�Ӧ�ȡ�H����285.8kJ/mol��3��726.5kJ/mol����130.9 kJ/mol��
��3�����ݷ�Ӧ��SO2(g)+1/2O2(g)  SO3(g)����CO(g)+1/2O2(g)
SO3(g)����CO(g)+1/2O2(g) CO2(g)��֪���٣��ڼ��õ���ӦSO2(g)+CO2(g)
CO2(g)��֪���٣��ڼ��õ���ӦSO2(g)+CO2(g)  SO3(g)+CO(g)����������ͬ�¶��·�ӦSO2(g)+CO2(g)
SO3(g)+CO(g)����������ͬ�¶��·�ӦSO2(g)+CO2(g)  SO3(g)+CO(g)ƽ�ⳣ��K��
SO3(g)+CO(g)ƽ�ⳣ��K�� ��
��
���㣺���鷴Ӧ�ȵļ��㡢�Ȼ�ѧ����ʽ����д�Լ�ƽ�ⳣ���ļ����


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�ϲ��еڶ���ѧ�߶���һ���¿���ѧ�Ծ����������� ���ͣ�������
�����10�֣����ס�������������ͬ���칹�壬�ڿ�����ȼ�յõ����������������ʱ����P4O6����������������P4O10��
��1����֪298Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
P4(s,����)+5O2(g)=P4O10(s) ��H1=" -2983.2" kJ?mol-1,
P(s������)+ 5/4O2(g)="1/4" P4O10(s) ��H2=" -738.5" kJ?mol-1
����¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ ��
��2����֪298Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4(s,����)+3O2(g)=P4O6(s) ��H= -1638kJ?mol-1����ij�ܱ������м���62g����50.4L��������״���£�����������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ ����Ӧ�����зų�������ΪΪ ��
��3����֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���µĻ�ѧ���ļ��ܣ�KJ��mol��:P-P 198��Cl-Cl 243��P-Cl 331��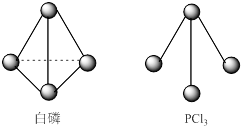
��ӦP4(s,����)+6Cl2(g)=4PCl3(s)�ķ�Ӧ�Ȧ�H = ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�ϲ��и߶���һ���¿���ѧ�Ծ��������棩 ���ͣ�������
�����10�֣����ס�������������ͬ���칹�壬�ڿ�����ȼ�յõ����������������ʱ����P4O6����������������P4O10��
��1����֪298Kʱ���ס�������ȫȼ�յ��Ȼ�ѧ����ʽ�ֱ�Ϊ��
P4(s,����)+5O2(g)=P4O10(s) ��H1= -2983.2 kJ•mol-1,
P(s������)+ 5/4O2(g)=1/4 P4O10(s) ��H2= -738.5 kJ•mol-1
����¶��°���ת��Ϊ�����Ȼ�ѧ����ʽΪ ��
��2����֪298Kʱ���ײ���ȫȼ�յ��Ȼ�ѧ����ʽΪP4(s,����)+3O2(g)=P4O6(s) ��H= -1638kJ•mol-1����ij�ܱ������м���62g����50.4L��������״���£�����������ʹ֮ǡ����ȫ��Ӧ�������õ���P4O10��P4O6�����ʵ���֮��Ϊ ����Ӧ�����зų�������ΪΪ ��
��3����֪����PCl3�ķ��ӽṹ��ͼ��ʾ�����ṩ���µĻ�ѧ���ļ��ܣ�KJ��mol��:P-P 198��Cl-Cl 243��P-Cl 331��

��ӦP4(s,����)+6Cl2(g)=4PCl3(s)�ķ�Ӧ�Ȧ�H = ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com