
����һ������ˮ��Һ��ֻ���ܺ������������е������֣�K����NH ��Cl����Mg2����Ba2����CO
��Cl����Mg2����Ba2����CO ��SO
��SO ����ȡ����100 mL��Һ��������ʵ�飺
����ȡ����100 mL��Һ��������ʵ�飺
(1)��һ�ݼ���AgNO3��Һ�г���������
(2)�ڶ��ݼ�����NaOH��Һ���Ⱥ��ռ�������0.04 mol��
(3)�����ݼ�����BaCl2��Һ�ø������6.27 g������������ϴ�ӡ������������Ϊ2.33 g��
��������ʵ�飬�����Ʋ���ȷ����(����)
A��K����һ������
B��100 mL��Һ�к�0.01 mol CO
C��Cl�����ܴ���
D��Ba2��һ�������ڣ�Mg2�����ܴ���
C��[�ɵ�����ʵ����֪n(BaSO4)��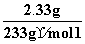 ��0.01 mol������Һ�к�0.01 mol��SO
��0.01 mol������Һ�к�0.01 mol��SO �����ɼ�����������������6.27 g����2.33 g֪����Һ�к���CO
�����ɼ�����������������6.27 g����2.33 g֪����Һ�к���CO ����n(CO
����n(CO )��
)��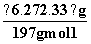 ��0.02 mol ������ʵ��(2)��֪ԭ��Һ�к���NH
��0.02 mol ������ʵ��(2)��֪ԭ��Һ�к���NH ��n(NH
��n(NH )��0.04 mol��������Һ�к���CO
)��0.04 mol��������Һ�к���CO ����Mg2�������ڣ����ں���SO
����Mg2�������ڣ����ں���SO ����Ba2�������ڣ�������Һ�е������ӾͿ���ΪNH
����Ba2�������ڣ�������Һ�е������ӾͿ���ΪNH ��K�������ݵ���غ㣬�����������������Ϊ0.01 mol��2��0.02 mol��2��0.06 mol������NH
��K�������ݵ���غ㣬�����������������Ϊ0.01 mol��2��0.02 mol��2��0.06 mol������NH �����������(0.04 mol��1��0.04 mol)����K��һ�����ڡ�����CO
�����������(0.04 mol��1��0.04 mol)����K��һ�����ڡ�����CO ��SO
��SO ������Ag���γɳ���������������ʵ�鲻��֤��һ����Cl��������ǿ��ܴ��ڡ�]
������Ag���γɳ���������������ʵ�鲻��֤��һ����Cl��������ǿ��ܴ��ڡ�]


 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й����л����������ȷ����(����)
A�����顢�ױ����״������Է���ȡ����Ӧ
B������ϩ�ɷ����ӳɷ�Ӧ
C�����ۡ���ά�ء������ʡ���֬�����ڸ߷��ӻ�����
D���Ҵ��������������ɫ�ķ�̪��Һ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ������Ԥ�ⲻ��ȷ���� (����)

A��ʵ������ã��ϲ���Һ��ɫ���ֲ���
B��ʵ�������KMnO4��Һ�г������ݣ�����ɫ����ȥ
C��ʵ�����ϡHNO3 Ƭ�̣���Һ�������ݲ��������ƿ�����ɺ���ɫ����ɫ
D��ʵ��������������Һ�����ɫ��ֹͣ���ȣ�������ͨ����ϵʱ�ɲ��������ЧӦ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���Ԫ�����ڱ���һ���֣��й�˵����ȷ���� (����)
| �� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| �� | c | d | |||||
| �� | a | b | e | f |
A��e���⻯���d���⻯���ȶ�
B��a��b��e����Ԫ�ص�ԭ�Ӱ뾶��e>b>a
C������Ԫ���У�cԪ�ص��ʵĻ�ѧ���������
D��c��e��f������������Ӧ��ˮ���������������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�������Թ�ҵ��ˮ�к���һ������Fe3����Cu2����Au3�������ӡ����������ͼ�еĹ������̣����ó��õ��ᡢ���ҵ�����еķ���м���ӷ�ˮ�л��ս𣬲�����һ���������������ͭ��

��д����հף�
(1)ͼ�б�Ŵ���������Ӧ���ʷֱ���
��____________����____________����____________��
��____________����____________��
(2)д���ٴ�������Ӧ�����ӷ���ʽ
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��
д���۴�������Ӧ�Ļ�ѧ����ʽ
________________________________________________________________________
________________________________________________________________________��
(3)����Ļ�ѧʽΪ________���ֱ�д�����������ͭ�ڹ�ҵ�ϵ�һ����Ҫ��;��
����___________________________________________________________________��
����ͭ_________________________________________________________________��
������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͼ��ʾ������������ȷ����(����)
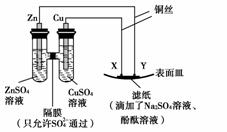
A��YΪ������������ԭ��Ӧ
B��XΪ����������������Ӧ
C��Y����ֽ�Ӵ�������������
D��X����ֽ�Ӵ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�߶���ѧ��ȤС��Ϊ��̽�����缫��ԭ����е����ã���Ʋ�����������һϵ��ʵ�飬ʵ�������¡��Ը����±��е�ʵ������ش��������⣺
| ��� | �缫���� | �������Һ | ������ָ��ƫת���� |
| 1 | Mg��Al | ϡ���� | ƫ��Al |
| 2 | Al��Cu | ϡ���� | ƫ��Cu |
| 3 | Al��ʯī | ϡ���� | ƫ��ʯī |
| 4 | Mg��Al | NaOH | ƫ��Mg |
| 5 | Al��Zn | Ũ���� | ƫ��Al |
(1)ʵ��1��2��Al�����ĵ缫�Ƿ���ͬ��________(��ǡ���)��
(2)ʵ��3�еĵ缫��Ӧʽ������______________________������______________________________������ܷ�Ӧ����ʽ______________________��
(3)ʵ��4��Al��________������缫��Ӧʽ��____________________________���жϵ缫��������_____________________________________________________________��
(4)����ʵ��5�е�����ָ��ƫ��Al��ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
CH4��H2��CO��ȼ���ȷֱ�Ϊ890.31kJ/mol��285.8kJ/mol��110.5 kJ/mol�������Ȼ�ѧ����ʽ��д��ȷ����
A��CH4(g)+2O2(g)=CO2(g)+2H2O(g) ��H=-890.31kJ/mol
B��2H2(g)+ O2(g)= 2H2O(l) ��H=-285.8kJ/mol
 C��CO (g)+ H2O(g)= CO2(g)+
C��CO (g)+ H2O(g)= CO2(g)+  H2 (g) ��H=+175.3kJ/mol
H2 (g) ��H=+175.3kJ/mol
D��2CO (g)+ O2(g) = 2CO2(g) ��H=-221 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͬ�����£�������Ϊ2 g��CO��H2�Ļ�����壬��O2����ȫȼ�պ�ȫ��ͨ��������Na2O2���壬������������� (����)
A��2 g B��1 g C��18 g D��������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com