
ʵ���ҳ����ü�ȩ���ⶨ(NH4)2SO4��Ʒ�е��������������䷴Ӧԭ��Ϊ��4NH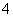 ��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1 mol(CH2)6N4H����1 mol H���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
��6HCHO===3H����6H2O��(CH2)6N4H��[�ζ�ʱ��1 mol(CH2)6N4H����1 mol H���൱]��Ȼ����NaOH����Һ�ζ���Ӧ���ɵ��ᡣij��ȤС���ü�ȩ������������ʵ�飺
�����ȡ��Ʒ1.500 g��
�������Ʒ�ܽ����ȫת�Ƶ�250 mL����ƿ�У����ݣ����ҡ�ȡ�
�������ȡ25.00 mL��Ʒ��Һ��250 mL��ƿ�У�����10 mL 20%�����Լ�ȩ��Һ��ҡ�ȡ�����5 min����1��2�η�̪��Һ����NaOH����Һ�ζ����յ㡣�����������������ظ�2�Ρ�
(1)���ݲ������գ�
�ټ�ʽ�ζ���������ˮϴ�Ӻ�ֱ�Ӽ���NaOH����Һ���еζ���������Ʒ�е�����������________(�ƫ�ߡ�����ƫ�͡�����Ӱ�족)��
����ƿ������ˮϴ�Ӻ�ˮδ��������ζ�ʱ��ȥNaOH����Һ�����________(�ƫ����ƫС������Ӱ�족)��
�۵ζ�ʱ�ߵα�ҡ����ƿ���۾�Ӧ�۲�________��
A���ζ�����Һ��ı仯
B����ƿ����Һ��ɫ�ı仯
�ܵζ��ﵽ�յ�ʱ����ָ̪ʾ����________ɫ���________ɫ��
(2)�ζ�������±���ʾ��
| �ζ� ���� | ������Һ�����/mL | ����Һ����� | |
| �ζ�ǰ�̶�/mL | �ζ���̶�/mL | ||
| 1 | 25.00 | 1.02 | 21.03 |
| 2 | 25.00 | 2.00 | 21.99 |
| 3 | 25.00 | 0.20 | 20.20 |
��NaOH����Һ��Ũ��Ϊ0.101 0 mol��L��1�������Ʒ�е�����������Ϊ________��
(1)��ƫ�ߡ�����Ӱ�졡��B�����ޡ��ۺ�(��dz��)��(2)18.85%
������(1)�ټ�ʽ�ζ���ϴ����δ�ñ�Һ��ϴֱ�Ӽ���NaOH��Һ���൱��ʹ��Һ��ϡ��ʹ�ⶨ���ƫ�ߣ�����ƿϴ�Ӻ�ˮδ�����������ı�Һ�������Ӱ�죻�۵ζ�ʱ�۾�Ӧע����ƿ����Һ����ɫ�仯���ܷ�̪��������������ɫ�������������Էۺ�ɫ(���ɫ)�����յ�ʱӦ����ɫ��ۺ�ɫ��
(2)���������Ϣ�����ƶ�n(N)��n(NH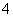 )��n(NaOH)���������εζ�����NaOH��Һ�����ƽ��ӦΪ20.00 mL������n(N)��0.101 0 mol��L��1��20.00��10��3 L��0.002 02 mol����Ʒ�е�����������Ϊ
)��n(NaOH)���������εζ�����NaOH��Һ�����ƽ��ӦΪ20.00 mL������n(N)��0.101 0 mol��L��1��20.00��10��3 L��0.002 02 mol����Ʒ�е�����������Ϊ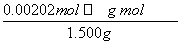 ��100%��18.85%��
��100%��18.85%��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 PTT�ǽ�������Ѹ�ٷ�չ���������������Ծ������ϣ������������ܣ�����Ϊ�������ϡ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�á���ϳ�·�߿����Ϊ��
PTT�ǽ�������Ѹ�ٷ�չ���������������Ծ������ϣ������������ܣ�����Ϊ�������ϡ���֯��ά�͵�̺�Ȳ��϶��õ��㷺Ӧ�á���ϳ�·�߿����Ϊ��
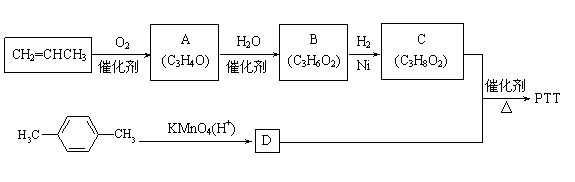
����A��B��C��Ϊ��״�����A�ܷ���������Ӧ��C��������1mol C���������Ʒ�Ӧ����22.4 L H2����״��������ش��������⣺
��1��A���������ŵ�����Ϊ ��B�Ľṹ��ʽΪ ��
��2��A����������Ӧ�Ļ�ѧ����ʽΪ ����Ӧ����Ϊ ��
��3������ʽΪC4H6O����A��Ϊͬϵ���ͬ���칹���� �֡�
��4����д����CH2=CHCH3Ϊ��Ҫԭ�ϣ����Լ���ѡ���Ʊ�CH3CH(OH)COOH�ĺϳ�·������ͼ��
��
 ���ϳ�·�߳��õı�ʾ��ʽΪ��M
���ϳ�·�߳��õı�ʾ��ʽΪ��M N����
N���� Ŀ����
Ŀ����
��֪��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A�㷺����Ϳ�ϡ���ī��������ҽҩ��ũҩ�м�������
��֪���л���A�������������£�
|
|
|
|
| �� �л���A�ĺ˴Ź�������ͼ����2�����շ壬�����֮����1�U1�� |
�ش��������⣺
��1��A����Է��������� ��
��2��A���й����ŵ�������___________��
��3��A������������Һ�ڼ��������·�Ӧ�Ļ�ѧ����ʽ��
��
��4��A�ж���ͬ���칹�壬������������Cu(OH)2���ȣ�������ɫ�������� �֣�
д����������һ��ͬ���칹��Ľṹ��ʽ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и�����Һ�����������Լ����Ϳ��Խ�����������(����)
A�����ᡢ�������ء�����ء�̼���
B�������ơ����ᡢ�Ȼ�李���������
C���Ȼ������Ȼ��ơ�����ء������
D���������ء�̼��ء����������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾ���ĸ�ԭ���װ�ã����н�����ȷ����(����)
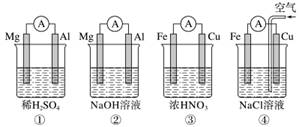
A���٢���Mg���������ۢ���Fe������
B������Mg���������缫��ӦʽΪ6H2O��6e��===6OH����3H2��
C������Fe���������缫��ӦʽΪFe��2e��===Fe2��
D������Cu���������缫��ӦʽΪ2H����2e��===H2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ԭ���ԭ�����ʵ�飬��֤Cu2����Fe3�������Ե�ǿ������д���缫��Ӧʽ������________________________________________________________________________��
����________________________________________________________________________��
���ڷ����ڻ���ʵ��װ��ͼ��Ҫ�����ձ������ţ���������·��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
2013��3���ҹ���ѧ�ұ�������ͼ��ʾ��ˮ��Һ����ӵ����ϵ�����������������(����)
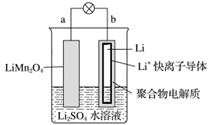
A��aΪ��ص�����
B����س�練ӦΪLiMn2O4===Li1��xMn2O4��xLi
C���ŵ�ʱ��a��﮵Ļ��ϼ۷����仯
D���ŵ�ʱ����Һ��Li����b��aǨ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ﮡ�������ͭ��������Ļ���������Cu4O(PO4)2����ͨ�����з�Ӧ�Ʊ���
2Na3PO4+4CuSO4+2NH3��H2O=Cu4O(PO4)2��+3Na2SO4+(NH4)2SO4+H2O
��1����̬Cu2+�ĺ�������Ų�ʽΪ ��
��2�����ݼ۲���ӶԻ���ģ�ͣ�VSEPR����PO43������������ԭ�Ӽ۲�ɼ����ӶԺµ��Ӷ�֮��Ϊ �����ӵĿռ乹���� ��
��3��Һ�����Ӽ����������룺2NH3 NH2��+NH4+��NH2���е�ԭ�ӹ�����ӻ������� ��H+����NH3�γ�NH4+����λ���Ľ����� ��
NH2��+NH4+��NH2���е�ԭ�ӹ�����ӻ������� ��H+����NH3�γ�NH4+����λ���Ľ����� ��
��4������CuSO4��5H2O�Ľṹʾ��ͼ���£��京�е������������� ��������ţ�
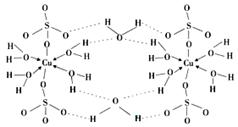
a�����Ӽ� b�����Լ� c�������� d����λ�� e����� f���Ǽ��Լ�
��5��������ͭ��Һ�м������KCN�����������[Cu(CN)4]2����Cu2+����λ��Ϊ ��1mol CN���к��еĦм�����ĿΪ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com