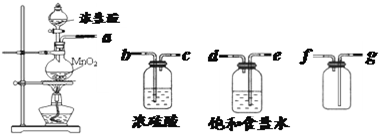
��2013?�㽭������Դ��һ����Ҫ�����Դ���������ֿɲ���H
2�Ļ�������ң���6.00g��������ȫ�ֽ⣬ֻ�õ�һ�ֶ�����Ԫ�صĽ������ʺ�6.72L��H
2��������ɱ�״����������ˮ��ӦҲ�ܷų�H
2��ͬʱ������һ�ְ�ɫ������ð�ɫ����������NaOH��Һ�����������ڴ��������¿ɷֽ�õ�H
2����һ�ֵ�������������ڱ�״���µ��ܶ�Ϊ1.25g?L
-1����ش��������⣺
��1���Ļ�ѧʽ��
AlH3
AlH3
���ҵĵ���ʽ��
��
��2������ˮ��Ӧ�Ļ�ѧ����ʽ��
AlH3+3H2O=Al��OH��3��+3H2��
AlH3+3H2O=Al��OH��3��+3H2��
��
��3������������þ��Ӧ�IJ�����
Mg3N2
Mg3N2
���û�ѧʽ��ʾ����
��4�����ڼ�����������CuO��Ӧ������Cu���������д���÷�Ӧ�Ļ�ѧ����ʽ
�������������Cu�п��ܻ�����Cu
2O�������ʵ�鷽����֤֮
ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����Cu2O
ȡ�����H2SO4�������Һ������˵�������к���Cu2O����֮����Cu2O
������֪��Cu
2O+2H
+�TCu+Cu
2++H
2O��
��5��������֮��
����
����
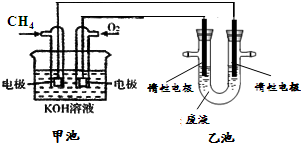
������ܡ������ܡ���������Ӧ����H
2���������
AlH3�е�HΪ-1�ۣ�NH3�е�HΪ+1�ۣ��п��ܷ���������ԭ��Ӧ��������
AlH3�е�HΪ-1�ۣ�NH3�е�HΪ+1�ۣ��п��ܷ���������ԭ��Ӧ��������
��

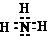
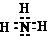
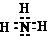 ���ʴ�Ϊ��AlH3��
���ʴ�Ϊ��AlH3��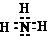 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
 ������������ȷ����
������������ȷ���� ���۵㣨���=������ԭ����
���۵㣨���=������ԭ����