
 1��2��3��4-���⻯���Ľṹ��ʽ��
1��2��3��4-���⻯���Ľṹ��ʽ�� ������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2?C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£�
������ʽ��C10H12��������Ϊ��ɫҺ�壬�д̼�����ζ���е�207�棬������ˮ����һ���������ܼ�������Һ�巢����Ӧ��C10H12+4Br2?C10H8Br4+4HBr�����ɵ����廯��������Ϊ��̬��������ˮ�����������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ��ʵ�鲽�����£����� �������������⻯���������Ʊ�������������Һ��ʵ��̽�����漰���ݷ�Ӧ��Һ����������廯���ӷ����ص�ѡ��Ӧװ��D����ʵ�飬ͨ���μӵ�Һ����ɫ������ȥ�жϷ�Ӧ��ȫ�������˷�Ӧ������ķ������ᴿ������ϲ�Ʒ��������������ԭ�ϵ���ȣ��������ԭ���غ���м��㣬�ݴ��жϽ��
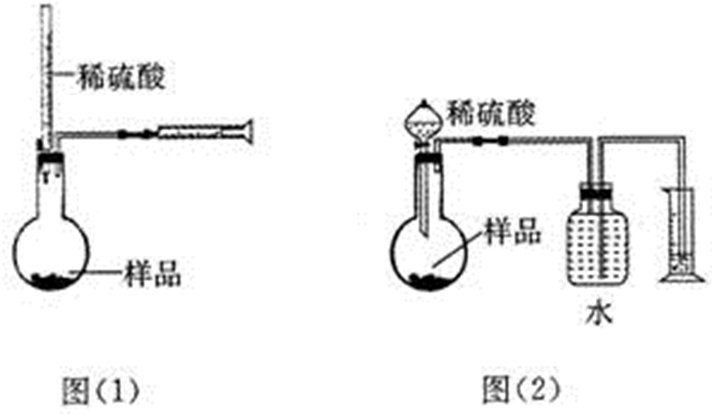
��1�����ݢ٢ڵIJ�����ʹ���Լ��ӷ����ص��жϺ�����װ�ã�
��2��Һ��������ɫ��������뵽��Ӧ�������������뷴Ӧ����ɫ�����������ȫ�������Һ�彫�Ա����ڷ�Ӧ�����ڣ�
��3�����ݲ������Ҫ�����˹�����Һ������жϣ�
��4���������廯��������Ϊ��̬�ͼ����������������ǣ�
��5������������ˮ��Һ�������������������66%��������Һ������Ϊ100g������Һ��HBr������Ϊ66g���ɼ���������ʵ������ٽ�Ϸ�Ӧԭ�����Ƴ����⻯�������ʵ������Ӷ��ɼ�������⻯����ˮ�������ȣ�
��� �⣺��1�����ղ���٣���ʵ���ǰ����⻯����ˮ�����ۼ���ͬһ�����з�Ӧ������B����ȷ������A�е�װ�ý��з�Ӧ���������ʹ̼�����ζ�����⻯��������Ⱦ������ͬ��Cװ���ڲ���ڷ�Ӧʱ��Ҳ����ɻ�����Ⱦ������A��C�����ԣ���ѡDװ�ã��ʴ�Ϊ��D��
��2�����淴Ӧ�Ľ��У��������ĺ��ɫҺ��������ɫ������Ӧ��ȫ�����Һ����ɫ������ȥ���ʴ�Ϊ�������Һ����ɫ�������ʣ�
��3�����ڲ���ڼ����˹�����Һ�壬������в����������⻯������ʣ����嵥�ʣ��ʴ�Ϊ������ȥ������Br2��
��4���������ɵ����廯��������Ϊ��̬�����Թ��˺�õ��Ĺ������������廯�������ۣ��ʴ�Ϊ�����廯�������ۣ�
��5������Һ������Ϊ100g������Һ��HBr������Ϊ66g��ˮ������Ϊ34g��HBr�����ʵ���$\frac{66g}{81g/mol}$=$\frac{22}{27}$mol������C10H12+4Br2?C10H8Br4+4HBr��֪���⻯�������ʵ���Ϊ$\frac{22}{27}$mol��4=$\frac{11}{54}$mol������Ϊ$\frac{11}{54}$mol��134g/mol=$\frac{737}{27}$g������������⻯����ˮ��������Լ��$\frac{737}{27}$g��36g��1��1.3���ʴ�Ϊ��1.3��
���� ���⿼���������⻯����Һ�塢����ˮ�ʹ�����Ϊԭ�ϣ��Ʊ�����������������Һ���漰��װ�õ�ѡ��ѧ����ʽ����д����ƽ����ֿ�����ѧ���ķ�������������������ѧ֪ʶ���յ������̶ȣ������Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ʵ����� | ���ӷ���ʽ |
| ����1��ȡ������Һ���Թ��У���������ữ��H2O2��Һ���� | 2Fe2++H2O2+2H+=2Fe3++2H2O |
| ����2����pH����3.7��8.6��ʹFe3+������ȫ�� | Fe3++3H2O?Fe��OH��3+3H+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��Ӧʱ��/min | n��CO��/mol | H2O/mol |
| 0 | 1.20 | 0.60 |
| t1 | 0.80 | |
| t2 | 0.20 |
| A�� | ��Ӧ��t1min�ڵ�ƽ������Ϊv��H2��=0.40/t1mol•L-1•min-1 | |
| B�� | �¶�����800�棬������Ӧƽ�ⳣ��Ϊ0.64��������ӦΪ���ȷ�Ӧ | |
| C�� | ���������������䣬��ƽ����ϵ����ͨ��0.20molH2O����ԭƽ����ȣ��ﵽ��ƽ��ʱCOת���ʼ�С | |
| D�� | ���������������䣬��ʼʱ�������г���0.60molCO��1.20 molH2O������ƽ��ʱ��n��CO2��=0.40 mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ñ���������Һ��ϴ��ʽ�ζ���2-3�� | |
| B�� | �ô����Һ��ϴ��ʽ�ζ���2-3�� | |
| C�� | �ô����Һ��ϴ��ƿ2-3�� | |
| D�� | ������ˮ��ϴ��ƿ2-3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com