
 ��֪A��B��C��D��E��F��Ϊǰ������Ԫ�أ�AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2��BԪ�ص�����������������Ӳ�����3����CԪ��ԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ�DԪ��ԭ�ӵ�M���Ӳ��P�ܼ�����3��δ�ɶԵ��ӣ�B������E���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����γ�E2B2��E2B�ͻ����FԪ��λ��Ԫ�����ڱ���ds������ԭ����Eԭ�Ӿ�����ͬ��������������
��֪A��B��C��D��E��F��Ϊǰ������Ԫ�أ�AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2��BԪ�ص�����������������Ӳ�����3����CԪ��ԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ�DԪ��ԭ�ӵ�M���Ӳ��P�ܼ�����3��δ�ɶԵ��ӣ�B������E���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����γ�E2B2��E2B�ͻ����FԪ��λ��Ԫ�����ڱ���ds������ԭ����Eԭ�Ӿ�����ͬ��������������| ������ | I1 | I2 | I3 | I4 | I5 | I6 | I7 |
| ��KJ��mol-1�� | 14.5 | 29.6 | 47.4 | 77.5 | 97.9 | 551.9 | 666.8 |
���� A��B��C��D��E��F��Ϊǰ������Ԫ�أ�BԪ�ص�����������������Ӳ�����3����BΪOԪ�أ�B������E���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Ӻ��е�����Ϊ10�����γ�E2B2��E2B�ͻ������EΪNaԪ�أ�CԪ��ԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ���CԪ�ؼ۵����Ų�Ϊ3s23p4����CΪSԪ�أ�DԪ��ԭ�ӵ�M���Ӳ��P�ܼ�����3��δ�ɶԵ��ӣ���DԪ�ؼ۵����Ų�Ϊ3s23p3����DΪPԪ�أ�FԪ��λ��Ԫ�����ڱ���ds������ԭ����Eԭ�Ӿ�����ͬ������������������������Ϊ1����F�ļ۵����Ų�Ϊ3d104s1��F��CuԪ�أ�AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2�����ڵڢ�A�壮
��1�������������ԭ����дCuԭ�Ӻ�������Ų���
��2�����������������ܼ�ȷ���������ڱ��е�����
��3��AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2����n=2ʱ��A��CԪ�أ�����Ԫ���γɵ���Է�������Ϊ26�ķ���Ϊ��Ȳ��
��4��AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2����n=3ʱ��A��SiԪ�أ�������γɵľ����Ƕ������辧�壬����ԭ�Ӿ��壻Si������ÿ��Siԭ��������4��Siԭ���γ���������ṹ��ÿ��Si-Si��ΪSiԭ���ṩ$\frac{1}{2}$�����ݾ�̯������Siԭ���������ۼ���Ŀ��
��5��ABn�ͷ���Aԭ�ӹ¶Ե�����=$\frac{1}{2}$��a-xb����aΪAԭ�Ӽ۵�����Ŀ��xΪAԭ�ӽ��ԭ������bΪBԭ������ϵ�����Ŀ��
Pԭ�Ӽ۲���Ӷ���=����+�¶Ե����������Pԭ�ӵŶԵ��ӣ�ȷ��PCl3���ӵĿռ乹�ͣ�
��6�����ݾ�̯�����㾧����Cuԭ�ӡ�Oԭ����Ŀ���ݴ�ȷ����ѧʽ�����ݾ�����ԭ����Ŀ���㾧������������V=$\frac{m}{��}$���㾧�������
��7����ͬ�ܲ���������������ܲ�����������ϴ�ʧȥ��ͬ�ܲ����ʱ�����ܻᷢ��ͻԾ���ɱ������ݿ�֪��ʧȥ��6������ʱ�������ܾ������ʸ�Ԫ������������Ϊ5��
��� �⣺A��B��C��D��E��F��Ϊǰ������Ԫ�أ�BԪ�ص�����������������Ӳ�����3����BΪOԪ�أ�B������E���Ӿ�����ͬ�ĵ��Ӳ�ṹ�����Ӻ��е�����Ϊ10�����γ�E2B2��E2B�ͻ������EΪNaԪ�أ�CԪ��ԭ�Ӻ����M����ֻ��2�ԳɶԵ��ӣ���CԪ�ؼ۵����Ų�Ϊ3s23p4����CΪSԪ�أ�DԪ��ԭ�ӵ�M���Ӳ��P�ܼ�����3��δ�ɶԵ��ӣ���DԪ�ؼ۵����Ų�Ϊ3s23p3����DΪPԪ�أ�FԪ��λ��Ԫ�����ڱ���ds������ԭ����Eԭ�Ӿ�����ͬ������������������������Ϊ1����F�ļ۵����Ų�Ϊ3d104s1��F��CuԪ�أ�AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2�����ڵڢ�A�壮
��1����������������֪��BΪ��Ԫ�أ�
��FΪCuԭ�ӣ����������Ϊ29�������������ԭ��������ļ۵����Ų�Ϊ3d104s1��
�ʴ�Ϊ������3d104s1��
��2����CΪSԪ�أ���Χ�����Ų�Ϊ3s23p4������������p�ܼ�������p��Ԫ�أ�
��EΪNaԪ�أ���Χ�����Ų�ʽΪ3s1������������s�ܼ�������s��Ԫ�أ�
�ʴ�Ϊ��p��s��
��3��AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2����n=2ʱ��A��CԪ�أ�����Ԫ���γɵ���Է�������Ϊ26�ķ���Ϊ��Ȳ����ṹʽΪH-C��C-H������ֱ���ͶԳƽṹ��������������������غϣ�Ϊ�Ǽ��Է��ӣ���Ȳ������C-H����Ϊ���Ҽ���-C��C-��������1���Ҽ���2���м�������Ȳ�����й�����3�Ҽ���2���м���
�ʴ�Ϊ���Ǽ��ԣ�3��2��
��4��AԪ�ص�ԭ�Ӽ۵����Ų�Ϊns2np2����n=3ʱ��A��SiԪ�أ�������γɵľ����Ƕ������辧�壬����ԭ�Ӿ��壻Si������ÿ��Siԭ��������4��Siԭ���γ���������ṹ��Siԭ�ӳ�4���Ҽ��������¶Ե��ӣ���ȡsp3�ӻ���ÿ��Si-Si��ΪSiԭ���ṩ$\frac{1}{2}$����ÿ��Siԭ�ӳɹ��ۼ���ĿΪ4��$\frac{1}{2}$=2���ʹ辧����Siԭ����Si-Si����Ŀ֮��=1��2��
�ʴ�Ϊ��ԭ�ӣ�sp3��1��2��
��5��PCl3�����У�����ԭ��P���й¶Ե��Ӷ���=$\frac{5-1��3}{2}$=1��PCl3������Sԭ�Ӽ۲���Ӷ���=3+1=4������ռ乹��Ϊ�����Σ�
�ʴ�Ϊ��1�������Σ�
��6���ɾ����ṹ��֪��������Cuԭ����Ŀ=4��Oԭ����Ŀ=1+8��$\frac{1}{8}$=2���ʸ�������Ļ�ѧʽΪCu2O����������Ϊ$\frac{4��64+16��2}{{N}_{A}}$g���þ�����ܶ�Ϊag•cm-3���������Ϊ$\frac{4��64+16��2}{{N}_{A}}$g��ag•cm-3=$\frac{288}{a{N}_{A}}$cm3��
�ʴ�Ϊ��Cu2O��$\frac{288}{a{N}_{A}}$��
��7����ͬ�ܲ���������������ܲ�����������ϴ�ʧȥ��ͬ�ܲ����ʱ�����ܻᷢ��ͻԾ���ɱ������ݿ�֪��ʧȥ��6������ʱ�������ܾ������ʸ�Ԫ������������Ϊ5������Ϊ����PԪ�أ�
�ʴ�Ϊ��P��
���� ���⿼�����ʽṹ�����ʣ���Ŀ�Ƚ��ۺϣ��漰�ṹ����λ�ù�ϵ����������Ų������ӽṹ�����ʡ��۲���ӶԻ������ۡ��ӻ����ۡ���ѧ���������ܡ���������ȣ����ؿ�������֪ʶ����Ҫѧ���߱���ʵ�Ļ���֪ʶ���Ѷ��еȣ���4��Ϊ�״��㡢�ѵ㣬ע��ʶ����ѧ��������ṹ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����Ҫ�ϳɻ�����A����ṹΪ��
�����Ҫ�ϳɻ�����A����ṹΪ�� ������˵����ȷ���ǣ�������
������˵����ȷ���ǣ�������| A�� | �ϳ�A��ԭ�Ͽ����ǣ�2-��Ȳ��2-��-1��3-����ϩ | |
| B�� | �ϳ�A��ԭ�Ͽ����ǣ���Ȳ��2��3-����-1��3-����ϩ | |
| C�� | �÷�Ӧ��ԭ����ȡ����Ӧ | |
| D�� | ������A��HBr �������ʵ���1��1�ӳ�ʱ�������ֲ�ͬ�IJ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������



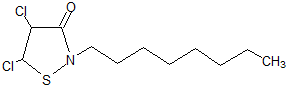 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ��������ʯ�͵�ʵ���У������ܵ�ˮ���������Ϸ���ˮ�·���ˮ | |
| B�� | ʯ���ѽⲻ��������ϩ����Ҫ���� | |
| C�� | ��֬��ʯ����ͬһ���л��� | |
| D�� | ��ʯ�ͷ���õ������ͣ���Ҫ�ɺ�5-12��̼ԭ�ӵ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����̫���ܡ�ˮ�ܡ����ܡ���ȼ��������Դ������ʹ��ú��ʯ�͵Ȼ�ʯȼ�� | |
| B�� | ��ú���С��������͡�Һ�������������ú���ۺ�����Ч�� | |
| C�� | �о���ú�������¼�������߲��������㹤ҵ�����Ŀ��ٷ�չ | |
| D�� | ʵ����Դ�ġ�3R�����ùۣ�����������Դ���ģ�Reduce����������Դ���ظ�ʹ�ã�Reuse������Դ��ѭ��������Recycle�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com