
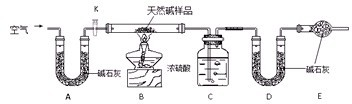
����ͼ��ʾװ��(�г�����ʡ��)����ʵ�飬��Һ��A��μ��뵽����B�У��ش��������⣺

(1)ͼ��Dװ����ʵ���е�������________��
(2)��AΪ70����H2SO4��Һ��BΪNa2SO3���壬Cʢ�к�I2�ĵ�����Һ������E���㹻����ʱ���C�е�����Ϊ________��C�з�����Ӧ�����ӷ���ʽΪ________��
(3)��AΪŨ��ˮ��BΪ��ʯ�ң�C��ʢ��AlCl3��Һ������E���㹻����ʱ���C�з�����Ӧ�����ӷ���ʽΪ________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ��ͷ�и�����ѧ�ڵ����ε��п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��ˮ����ͭ��ǿ���»ᷢ���ֽⷴӦ��
CuSO4  CuO
+ SO3�� 2SO3
CuO
+ SO3�� 2SO3  2SO2��+
O2��
2SO2��+
O2��
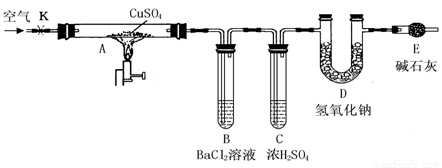
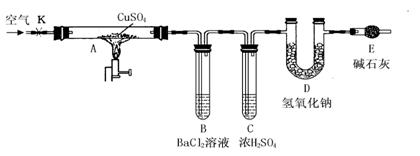
����ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������

ʵ�鲽�裺
�ٳ�����ӦǰD�ܵ�������
�����Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ���ȡ�
�۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ����ѳ�ȥ������̼����������Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
�ش��������⣺
��1����Ӧ2SO3(g)  2SO2(g) + O2(g)
��ƽ�ⳣ������ʽΪK=
��
2SO2(g) + O2(g)
��ƽ�ⳣ������ʽΪK=
��
��2��B���г��¶����������⣬���ɿ����������� �����¶��������ߵ���Ҫԭ���� ��B���з�����Ӧ���й����ӷ���ʽ�� ��
��3������E�������� ��
��4������������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m������ֽ��˵���ˮCuSO4��������(��ѡ��һ�ش�)
������ܣ���ֽ����ˮCuSO4������Ϊ ����m��ʾ����
��������ܣ���ԭ���� ��Ϊ���ܲ�÷ֽ��˵���ˮ����ͭ����������ļ�ʵ�鷽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ����4��˫����ϰ��ѧ�Ծ��������棩 ���ͣ�ʵ����
��13�֣���ˮ����ͭ��ǿ���»ᷢ���ֽⷴӦ��
CuSO4  CuO + SO3��
2SO3
CuO + SO3��
2SO3  2SO2��+ O2��
2SO2��+ O2��
����ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������

ʵ�鲽�裺
�ٳ�����ӦǰD�ܵ�������
�����Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ���ȡ�
�۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ����ѳ�ȥ������̼����������Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
�ش��������⣺
(1) 2SO3(g)  2SO2(g) + O2(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
2SO2(g) + O2(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
(2) B���г��¶����������⣬���ɿ����������� �����¶��������ߵ���Ҫԭ���� ��B���з�����Ӧ���й����ӷ���ʽ�� ��
(3)����E�������� ��
(4)����������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m������ֽ��˵���ˮCuSO4��������(��ѡ��һ�ش�)
������ܣ���ֽ����ˮCuSO4������Ϊ ����m��ʾ����
��������ܣ���ԭ���� ��Ϊ���ܲ�÷ֽ��˵���ˮ����ͭ����������ļ�ʵ�鷽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ������ѧ�ڿ�ѧ������⻯ѧ�Ծ� ���ͣ�ʵ����
��13�֣���ˮ����ͭ��ǿ���»ᷢ���ֽⷴӦ��
CuSO4  CuO + SO3��
2SO3
CuO + SO3��
2SO3  2SO2��+ O2��
2SO2��+ O2��
����ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������
ʵ�鲽�裺
�ٳ�����ӦǰD�ܵ�������
�����Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ���ȡ�
�۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ����ѳ�ȥ������̼����������Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
�ش��������⣺
(1) 2SO3(g)  2SO2(g) + O2(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
2SO2(g) + O2(g)
�÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
(2) B���г��¶����������⣬���ɿ����������� �����¶��������ߵ���Ҫԭ���� ��B���з�����Ӧ���й����ӷ���ʽ�� ��
(3)����E�������� ��
(4)����������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m������ֽ��˵���ˮCuSO4��������(��ѡ��һ�ش�)
������ܣ���ֽ����ˮCuSO4������Ϊ ����m��ʾ����
��������ܣ���ԭ���� ��Ϊ���ܲ�÷ֽ��˵���ˮ����ͭ����������ļ�ʵ�鷽���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ��ͨ��ͨ���������ص��ȵ�ר���⻯ѧ�Ծ� ���ͣ�ʵ����
��15�֣���ˮ����ͭ��ǿ���»ᷢ���ֽⷴӦ��
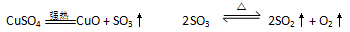
����ͼ��ʾװ�ã��г���������ȥ��������D���ڷ�Ӧǰ��������������ֽ��˵���ˮ����ͭ��������
ʵ�鲽�裺
�ٳ�����ӦǰD�ܵ�������
�����Ӻ�װ�ã��ر�K������Ӳ�ʲ�����Aһ��ʱ���ֹͣ���ȡ�
�۴�Ӳ�ʲ�����A��ȴ��K��ͨ��һ��ʱ����ѳ�ȥ������̼����������Ŀ�����
���ٳ���D�ܣ����䷴Ӧǰ���������Ϊm��
�ش��������⣺
(1) 2SO3(g) 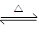 2SO2(g)
+ O2(g) �÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
2SO2(g)
+ O2(g) �÷�Ӧ��ƽ�ⳣ������ʽΪK�� ��
(2) B���г��¶����������⣬���ɿ����������� �����¶��������ߵ���Ҫԭ���� ��B���з������й����ӷ���ʽ�� ��
(3)�������ͨһ��ʱ���ѳ�ȥ������̼����������Ŀ�����Ŀ���� ��
(4)����������ʵ�飬����B��C��D����������վ���ȫ�������Կ�����CO2��Ӱ�죬�ܷ����m������ֽ��˵���ˮCuSO4��������(��ѡ��һ�ش�)
������ܣ���ֽ����ˮCuSO4������Ϊ ����m��ʾ����
��������ܣ���ԭ���� ��Ϊ���ܲ�÷ֽ��˵���ˮ����ͭ����������ļ�ʵ�鷽���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com