
����Ŀ��ij100mL���Һ�У�HNO3��H2SO4�����ʵ���Ũ�ȷֱ�Ϊ0.1mol/L��0.4mol/L����û��Һ�м���1.92gͭ�ۣ�����ʹ��Ӧ������ȫ������˵����ȷ����(���Է�Ӧǰ����Һ����仯)�� ��
A.������Һ��c(Cu2+)=0.225mol/L
B.������Һ��c(H+)=0.5mol/L
C.���������ڱ�״���µ����Ϊ0.448L
D.��Ӧ��ת��0.06mol�ĵ���
���𰸡�B
��������
n(Cu)=![]() =0.03mol����Һ��n(H+)=0.1mol/L��0.1L+0.4mol/L��0.1L��2=0.09mol��n(NO3)=0.1mol/L��0.1L=0.01mol��������Һ����ͭ�ۺ�����Ӧ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���������ӷ�Ӧ����ʽ��֪NO3�������㣬��Ӧ��ͭ�����ʵ���Ϊ0.015mol�����ĵ������ӵ����ʵ���Ϊ0.04mol��
=0.03mol����Һ��n(H+)=0.1mol/L��0.1L+0.4mol/L��0.1L��2=0.09mol��n(NO3)=0.1mol/L��0.1L=0.01mol��������Һ����ͭ�ۺ�����Ӧ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O���������ӷ�Ӧ����ʽ��֪NO3�������㣬��Ӧ��ͭ�����ʵ���Ϊ0.015mol�����ĵ������ӵ����ʵ���Ϊ0.04mol��
A. ��Ӧ��ͭ�����ʵ���Ϊ0.15mol������c(Cu2+)=![]() =0.15mol/L����A����
=0.15mol/L����A����
B. ��Ӧ���ĵ������ӵ����ʵ���Ϊ0.04mol����ʣ���n(H+)=0.09mol-0.04mol=0.05mol����Һ���Ϊ100mL������Ũ��Ϊ0.5mol/L����B��ȷ��
C. �������ӷ���ʽ��֪���ɵ�����n(NO)= n(NO3)=0.01mol����������Ϊ0.224L����C����
D. ��Ӧ������0.01mol NO3����ԭ��NO��ת�Ƶ��ӵ����ʵ���Ϊ0.03mol����D����
�ʴ�ΪB��


 ���Ž�����ٰθ��νӹ㶫���������ϵ�д�
���Ž�����ٰθ��νӹ㶫���������ϵ�д� �����������ҵ�������������ϵ�д�
�����������ҵ�������������ϵ�д� ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����з�Ӧ�����ӷ���ʽ��д��ȷ����
A. �����ǻ�����������Һ�еμӹ�����̼��������Һ��[Al(OH)4]��+4H��=Al3++2H2O
B. ������SO2����ͨ��������NaClO��Һ�У�SO2��2ClO����H2O=SO32-��2HClO
C. NaHSO4��Һ��Ba(OH)2��Һ��Ӧ�����ԣ�2H��+SO42-+Ba2++2OH��=BaSO4��+2H2O
D. ���ˮ�еμӱ����Ȼ�����Һ��Fe3++3H2O=Fe(OH)3��+3H��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1��25 ��ʱ���Ʊ������������漰���Ȼ�ѧ����ʽ��ƽ�ⳣ�������
�Ȼ�ѧ����ʽ | ƽ�ⳣ�� | |
�� | 2NO2(g)+NaCl(s) | K1 |
�� | 4NO2(g)+2NaCl(s) | K2 |
�� | 2NO(g)+Cl2(g) | K3 |
����¶��£���H3=_______________kJmol-1��K3=_____________����K1��K2��ʾ����
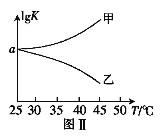
��2��25��ʱ�������Ϊ2L�ĺ����ܱ�������ͨ��0.08 mol NO��0.04 molCl2����������Ӧ�ۣ�����Ӧ��ʼ�����ʱ�¶���ͬ������ѹǿ����ʾ��Ӧ������ѹǿ(p)��ʱ��(t)�ı仯��ͼ��ʵ����ʾ����H3 ___���>����<����=����0��������������ͬ�����ı�ijһ�����������ѹǿ��ʱ��ı仯��ͼ��������ʾ����ı��������_____________����5 minʱ���ٳ���0.08 mol NO��0.04 molCl2�����������ƽ����Է���������_____________�����������С�����䡱����ͼ���Ǽס�����ͬѧ���������Ӧ�۵�ƽ�ⳣ���Ķ���ֵ��lgK�����¶ȵı仯��ϵͼ��������ȷ��������______����ס����ҡ�����aֵΪ__________��25 ��ʱ��÷�Ӧ����ijʱ�̣�NO(g)��Cl2(g)��NOCl(g)��Ũ�ȷֱ�Ϊ0.8��0.1��0.3�����ʱv��_________v�����>����������=����

(3)��300 �桢8 MPa�£���CO2��H2�����ʵ���֮��1��3 ͨ��һ�ܱ������з���CO2(g)��3H2(g)![]() CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
CH3OH(g)��H2O(g)�з�Ӧ���ﵽƽ��ʱ�����CO2��ƽ��ת����Ϊ50%����÷�Ӧ�����µ�ƽ�ⳣ��ΪKp��_____(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��ӦN2(g)+3H2(g)![]() 2NH3(g)��ij�¶��£�������̶���1L�ܱ������г���1mol N2(g)��3mol H2(g)����ò�ͬʱ�̷�Ӧǰ���ѹǿ��ϵ���±���ʾ��
2NH3(g)��ij�¶��£�������̶���1L�ܱ������г���1mol N2(g)��3mol H2(g)����ò�ͬʱ�̷�Ӧǰ���ѹǿ��ϵ���±���ʾ��
ʱ��/min | 5 | 10 | 15 | 20 | 25 | 30 |
ѹǿ��ֵP��/Pǰ | 0.98 | 0.88 | 0.80 | 0.75 | 0.75 | 0.75 |
(1)0~15min��,��H2��ʾ��ƽ����Ӧ����Ϊv(H2) =___________________mol��L-1��min-1��
(2)�ﵽƽ��ʱN2��ת����Ϊ________�����¶��µ�ƽ�ⳣ��Ϊ___________(������λС��)��
(3)��֪�÷�ӦΪ���ȷ�Ӧ����ͼΪ��ͬ�����·�Ӧ������ʱ��ı仯���(ÿ�ν��ı�һ������)��aʱ�ı������������____________��bʱ�ı������������_______________��

(4)һ�������µ��ܱ������У��÷�Ӧ�ﵽƽ���Ҫ���H2��ת���ʣ����Բ�ȡ�Ĵ�ʩ����_________��
A�����µ�ѹ B��������� C������N2��Ũ�� D������H2��Ũ�� E�������NH3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���أ�31Ga����һ����Ҫ����Ԫ�أ��ؼ��仯�����ڵ��ӹ�ҵ������ӹ�ҵ��������ҵ�ͳ������ϵ��������Ź㷺��Ӧ�á��ش��������⣺
��1����̬Gaԭ��ռ������ܼ����ӵĵ���������ͼ��״Ϊ__________��δ�ɶԵ�����Ϊ________________��
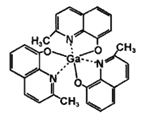
��2��Ga(NO3)3�������ӵ����幹����_____________��д��һ����������ӵ����幹����ͬ�ķ��ӵĻ�ѧʽ___________��
��3��2-��-8-�ǻ�����أ���ͼ��Ӧ���ڷ���ӡ��������2-��-8-�ǻ������������Ԫ�ص縺���ɴ�С��˳����____________________________����Ԫ�ط���)���ṩ�µ��ӶԵijɼ�ԭ����_____________��
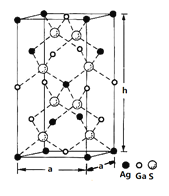
��4��һ�ֹ��ذ뵼����ϵľ����ṹ��ͼ��ʾ�����ء����γɵĻ�����ľ����ǵ���Ϊ�����εij����壬�ṹ����ͼ��ʾ����þ����������λ��Ϊ___________����������ı߳�a=5.75 nm����h=10.30nm���þ����ܶ�Ϊ__________________g��cm-3���г�����ʽ���ɣ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʯ����ȡ�طʺ�������������Ҫԭ�ϡ�����ʯ����ɺ��������ƣ�����������������������������ʡ�����ʵ�鲽����ͼ��ʾ��
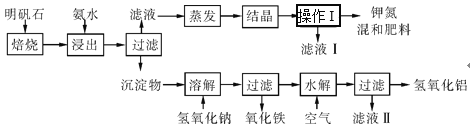
��������ͼʾ�����������ա�
��1������ʯ���պ���ϡ��ˮ������ʵ����Ҫ500mLϡ��ˮ(ÿ������19.60g��)��ҪȡŨ��ˮ(ÿ������250.28g��)___mL���ù��Ϊ___mL��Ͳ��ȡ��
��2��д�����������������ʵĻ�ѧʽ��___��
��3���������������___�����õIJ���������___��
��4��Ϊ�ⶨ��Ϸ���K2SO4��(NH4)2SO4�мصĺ���(��K2O��)���������в��裺
�ٳ�ȡ�ص�������������ˮ����������BaCl2��Һ������___��
��___��___��___(������дʵ���������)��
����ȴ�����ء�
��������Ϊmg�����������ʵ���Ϊnmol���������мصĺ���(��K2O��)Ϊ___%������������(�ú�m��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��FΪԪ�����ڱ���ǰ������Ԫ�أ���ԭ��������������A����������Ԫ�ؼȲ�ͬ����Ҳ��ͬ���壬B��һ�ֺ����ڿ���ʱ����������һЩ����������C���������ǵ����������Ҫ����֮һ��Dԭ�Ӻ��������8�ֲ�ͬ���˶�״̬��E�Ļ�̬ԭ����ǰ������Ԫ�صĻ�̬ԭ���е���������࣬FԪ�صĻ�̬ԭ�������ܲ�ֻ��һ�����ӣ������ܲ���ѳ������ӡ�
��1��д����̬Eԭ�ӵļ۵����Ų�ʽ_______��
��2��A��C���γ�CA3���ӣ��÷�����Cԭ�ӵ��ӻ�����Ϊ______���÷��ӵ�����ṹΪ_____��C�ĵ�����BD�������ǵȵ����壬�ݵȵ������ԭ����д��BD������ĵ���ʽ______��A2D��Һ̬�γɾ���ʱ�ܶȼ�С������Ҫԭ����__________����������������
��3����֪D��F���γ�һ�ֻ�����侧���Ľṹ��ͼ��ʾ����û�����Ļ�ѧʽΪ___��������Dԭ�Ӻ�Fԭ�Ӽ�ľ���Ϊa cm�������ӵ�������ֵΪ![]() NA����þ�����ܶ�Ϊ______g��cm��3���ú�a��NA��ʽ�ӱ�ʾ����
NA����þ�����ܶ�Ϊ______g��cm��3���ú�a��NA��ʽ�ӱ�ʾ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ݻ�һ�����ܱ������У�����һ������ NO(g)������C(s)��������Ӧ C(s)��2NO(g)![]() CO2(g)��N2(g)��ƽ��״̬ʱ NO(g)�����ʵ���Ũ�� c(NO)���¶� T �Ĺ�ϵ��ͼ��ʾ��������˵������ȷ����( )
CO2(g)��N2(g)��ƽ��״̬ʱ NO(g)�����ʵ���Ũ�� c(NO)���¶� T �Ĺ�ϵ��ͼ��ʾ��������˵������ȷ����( )
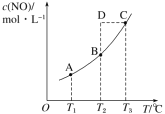
A.�÷�Ӧ�� ��H>0B.���÷�Ӧ�� T1��T2 ʱ��ƽ�ⳣ���ֱ�Ϊ K1��K2���� K1<K2
C.�� T3 ʱ�������������ܶȲ��ٱ仯��������жϷ�Ӧ�ﵽƽ��״̬ CD.�� T2 ʱ������Ӧ��ϵ����״̬D�����ʱһ���� v ��<v ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��H2A�Ƕ�Ԫ���ᣬ25��ʱ������һ��c(H2A)��c(HA��)��c(A2��)��0.1mol��L-1��H2A��NaOH�����Һ����Һ��H2A��HA����A2����ռ�����������������ʵ�����������������ҺpH�仯�Ĺ�ϵ��ͼ��ʾ������˵����ȷ����
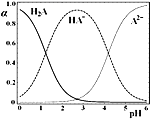
A.c(Na��)��0.1mol��L-1����Һ�У�c(H��)��c(A2��)��c(OH��)��c(H2A)
B.c (HA��)��c(A2��)����Һ�У�c(Na��)��3c(A2��)
C.c (HA��)��0.5mol��L-1����Һ�У�2c(H2A)��c(H��)��c(OH��)��1.5mol��L-1
D.pH��2����Һ�У�c(HA��)��2c(A2��)��0.1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com