
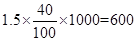 ��4�֣�
��4�֣�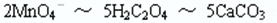
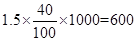 mg��
mg��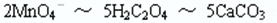



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
 ��
�� ��ɵĻ����18.400 g,��100 mL���ᷴӦ��(�����漰�����������Ϊ��״����,���ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)
��ɵĻ����18.400 g,��100 mL���ᷴӦ��(�����漰�����������Ϊ��״����,���ʱ�����ô���ĸ��ʽ�ӱ�ʾ��)  ��
�� �������ֱ�Ϊ �� ��
�������ֱ�Ϊ �� �� ��
�� �����Ϊ L��
�����Ϊ L�� �����,����Ҫ֪�� ��
�����,����Ҫ֪�� �� ��
�� �����Ե����ʵ������,��18.4 g����������������������ȫ��Ӧʱ����
�����Ե����ʵ������,��18.4 g����������������������ȫ��Ӧʱ���� �����ʵ���(��n��ʾ)�ķ�Χ�� ��
�����ʵ���(��n��ʾ)�ķ�Χ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��80mL | B��100mL | C��115mL | D��145mL |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
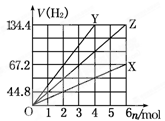
| A��n(Na)��n(Fe)��2��1���� | B��n(Mg)��n(K)��1��2 |
| C��n(Na)��n(Al)��1��3 | D��n(K)��n(Al)��1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1.0 L 1 mol��L��1��ˮ�У�NH3��H2O�ķ�����Ϊ6.02��1023 |
| B������7.1 g Cl2��ˮ��ȫ��Ӧʱת�Ƶĵ�����Ϊ0.1��6.02��1023 |
| C��������(P4)����������ṹ��31 g�����к���P��P������Ϊ6��6.02��1023 |
| D��20 g��ˮ(2H216O)�к��е�������Ϊ8��6.02��1023 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NaOH��Ħ������Ϊ40g |
| B��1mol O2��������������Է���������� |
| C��1mol OH ��������Ϊ17g/ mol |
| D����������������λg/ mol������ֵ�ϵ����������ԭ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��1:1 | B��1:3 | C��91:94 | D��1:94 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com