
MnO2��һ����Ҫ�Ĵ�����ij�о���ѧϰС������˽���MnO2�����н϶��MnO��MnCO3����Ʒת��Ϊ��MnO2ʵ�飬���������£�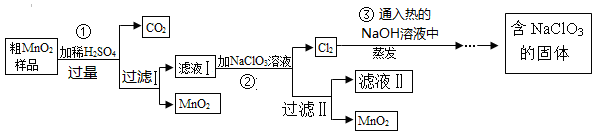
��1��д��1���ö��������������Ļ�ѧ��Ӧ����ʽ ��
��2���ڢڲ���Ӧ�����ӷ�Ӧ����ʽΪ ��
��3��������ˢ����õ�MnO2�Ƿ�ϴ�Ӹɾ��ķ����� ��
��4���ڢ۲���Ӧ�Ļ�ѧ��Ӧ����ʽΪ ��
��5�����������п�����ѭ��ʹ�õ������� ���ѧʽ�������˲��������������ж�Ҫ�õ��IJ��������� ��
��6������MnO2��Ʒ������Ϊ25.38g���ڢٲ���Ӧ�����˵õ�17.4g MnO2�����ռ���0.448LCO2����״���£�������Ʒ��������MnO����Ϊ g��
��1��2KClO3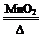 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2 2H2O��O2��
2H2O��O2��
��2��5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+
��3��ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ�
��4��3Cl2+ 6NaOH NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
NaClO3 + NaCl + 3H2O ��5��NaClO3������������1�֣� ��6��5. 68g
���������������1��ʵ��������ȡ����ʱ���ö�����������������Ӧ�Ļ�ѧ����ʽΪ2KClO3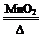 2KCl��3O2����2H2O2
2KCl��3O2����2H2O2 2H2O��O2����
2H2O��O2����
��2������ؾ���ǿ�����ԣ��ܰ���Һ�е�Mn2+�������ɶ������̣�������صĻ�ԭ��������������Ӧ�����ӷ���ʽΪ5Mn2++2ClO3-+4H2O��Cl2��+5MnO2+8H+��
��3����Һ�к���SO42��������������������SO42������˿���ͨ������SO42����������������Ƿ�ϴ�Ӹɾ���������ȷ��ʵ�������ȡ���һ��ϴ��Һ���Թ��У��μ��Ȼ�����Һ��������������֣�˵����ϴ�Ӹɾ���
��4���������ȵ�����������Һ��Ӧ���������ơ��Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ3Cl2+ 6NaOH NaClO3 + NaCl + 3H2O��
NaClO3 + NaCl + 3H2O��
��5���ɹ�������ͼ���Կ�����ѭ�����õ���NaClO3����ǰ����Ϊ��Ӧ����˺��������������������ʿ���ѭ��ʹ�á����˲��������������ж�Ҫ�õ��IJ��������Dz�������
��6����״����CO2�����ʵ�����0.448L��22.4L/mol��0.02mol�������̼ԭ���غ��֪̼���̵����ʵ�����0.02mol��������0.02mol��115g/mol��2.3g����ԭ�������MnO��������25.38g��17.4g��2.3g��5.68g��
���㣺���������Ʊ������ʵķ������ᴿ�����ʺ����ļ����Լ�ʵ�鷽����Ƶ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ˮ����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ���ɵ�����ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO3��SO2��O2�е�һ�֡����ֻ����֡�ij��ѧ����С�����̽����ʵ�飬�ⶨ��Ӧ������SO3��SO2��O2�����ʵ�����������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ���õ�����������ͼ��ʾ��
��������롿
����I������ͭ���ȷֽ���������ijɷֿ���ֻ��SO3һ�֣�
���������ͭ���ȷֽ���������ijɷֿ���ֻ��_______���֡�
���������ͭ���ȷֽ���������ijɷֿ��ܺ���_______���֡�
��ʵ��̽����
��֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣
��1����װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ�١��������ޡ��ݡ�____��_____��_____��______���ڡ�(��ӿ����)
��2����ʵ�����ʱװ��B����Ͳû���ռ���ˮ����֤������_______(�I������)��ȷ��
��3��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ��ʵ���������������Ҳ��
ͬ���������£�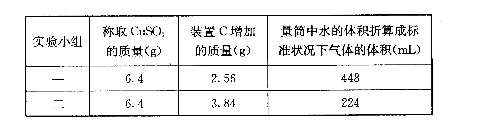
��ͨ�����㣬�ƶϳ��ڵ�һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ����ʽ��
��һС�飺_____________________________________________________________;
�ڶ�С�飺_____________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
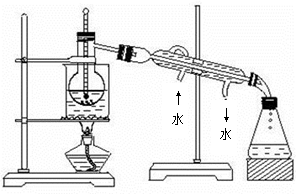
��Ԫ�ض��˵Ľ����dz���Ҫ�������ǴӺ������з�������ʵ⣨I2��������ͼ��
��1�����̢ٵ�ʵ�����������____________��____________��
��2�����������У�����������ԭ��Ӧ����_______�����ӷ���ʽΪ ��
��3�����̢۵�ʵ�����������___________��___________���ڴ˹����У��ɹ�ѡ�����
���ܼ���_______������ĸ���ţ���
| A���ƾ� | B��CCl4 | C���� | D������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ij��ѧ��ȤС������ij����������ͭп����ȡ����ZnOʵ���������£�
��ش��������⣺
��1���������ۺ�����Ӧ�����ӷ���ʽΪ_________________________________��
��2���ס�����ͬѧѡ���������������ò�ͬ�ķ�������ȡ������
A B
�ټ�ͬѧʹ�õ�ҩƷ����ʯ�����Ȼ�泥���Ӧѡ��װ��_______����дװ�ô��ţ������ɰ����Ļ�ѧ����ʽΪ_______________________________________��
����ͬѧѡ����װ��B����ʹ�õ�����ҩƷ������Ϊ_______________��
��3��H2O2��������____________________________________________________��
��4�����������еõ���Fe(OH)3����KClO��Һ�ڼ��Ի������������õ�һ�ָ�Ч�Ķ��ˮ��������K2FeO4�����÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ____________��
��5����֪��Һa�к���CO32-��SO42-������������ӣ���ֻ����ȡ��һ����Ʒ�������������Ӵ��ڵ�ʵ���������Ϊ________________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ʵ������ȡ��ϩ�ķ�Ӧԭ��Ϊ��CH3CH2OH CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������
CH2=CH2����H2O����Ӧʱ�������¶ȹ��߶�ʹ�Ҵ���ŨH2SO4��Ӧ����������SO2�������������ʵ��ȷ�������������������ϩ�Ͷ�������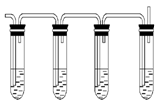
�Իش��������⣺
��1��ͼ�Т٢ڢۢ�װ��ʢ�ŵ��Լ��ֱ��ǣ���ѡ����ĸ������_________����_________��
| A��Ʒ����Һ | B��NaOH��Һ | C��Ũ���� | D�����Ը��������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�屻��Ϊ������Ԫ�ء�����֪Br2�ķе�Ϊ59�棬����ˮ���ж��Ժ�ǿ��ʴ�ԡ�ʵ����ģ��Ӻ�ˮ����ȡ�����Ҫ����Ϊ��
����1������ˮ����Ũ����ȥ����
����2������ȥ���κ��ĸҺ�ữ��ͨ��������������ʹBr��ת��ΪBr2��
����3������2����ˮ��Һ��ͨ���ȿ�����ˮ���������嵥�ʴ���ʢ�ж�������ˮ��Һ��������
����4�������������ͨ��������������ʹBr��ת��ΪBr2
����5�������Ȼ�̼��ȡ�嵥�ʣ�����Һ������ô��塣
��1������3�еķ�Ӧ�����ӷ���ʽ ��
��2������2���Ѿ��Ƶ����壬��Ҫ���в���3�Ͳ���4��ԭ���� ��Ԫ�ء�
��3������5����ȡ�ͷ�Һ����Ҫ����Ҫ��������Ϊ ��
��4��������ͼʵ��װ�þ��ƴ��塣
��ͼ����ȴˮӦ��B�� �ڽ���(�a����b��) ��
��C�мӱ���Ŀ���ǽ��£�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�������ƾ��壨CaO2��8H2O���ʰ�ɫ������ˮ��������350�����ҿ�
ʼ�ֽ�ų��������������ƿ����ڸ��Ƶر�ˮ�ʡ��������ؽ������ӷ�ˮ��Ӧ����
���ȡ�ʵ���ҿ��ù�ҵ̼��ƣ���MgCO3��FeCO3�����ʣ���ȡ������̼��ƣ�Ȼ����
�ô���̼�����ȡ�������ƣ�����Ҫ�������£�
�ش��������⣺
�����������漰�Ļ�ѧ��Ӧ����������ԭ��Ӧ���� ������д����������һ�������ӷ���ʽ�� ��
��2����Ӧ������CaO2��8H2O�Ļ�ѧ��Ӧ����ʽΪ ��
��Ӧʱ�ñ�ˮ��ȴ����Ҫԭ���� ��
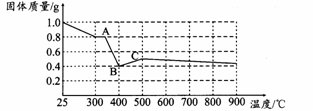
��3�����������ƾ����������м��Ȳ��������¶ȡ������Ʒ�������¶ȵı仯��ͼ������ʾ����350���Ժ����ù������ʵĻ�ѧʽΪ ��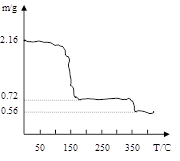
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������(FeC2O4��2H2O)�ʵ���ɫ��ij������Ϊ̽������ҵ������Ļ�ѧ���ʣ� ������һϵ��ʵ��̽����
(1)��ʢ�в�������������Թ��е��뼸�������ữ��KMnO4��Һ����������Һ��ɫ��Ϊ�ػ�ɫ������������̼�������ɡ���˵����������������� (������ԡ�������ԭ�ԡ����ԡ�)������Ӧ������1 mol FeC2O4��2H2O����μӷ�Ӧ��KMnO4Ϊ mol��
(2)���ϱ��������ܱ������м��ȵ�һ���¶�ʱ�����������������ȫ�ֽ⣬���ɼ��������������Ϊ��ɫ���塣��������ݿα��������ܵ���������������ʣ��Ժ�ɫ��������������¼��裬������ɼ�����ͼ�������
����һ��ȫ����FeO
�������
��������
(3)Ϊ��֤��������һ�Ƿ��������������������о���
�������о�����������±������ݡ�
| ʵ�鲽��(��Ҫ��д�������������) | Ԥ��ʵ������ͽ��� |
| ȡ������ɫ���壬 | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ����ƽ�VOSO4�е�K2SO4��SiO2���ʳ�ȥ�����յõ�V2O5���������£�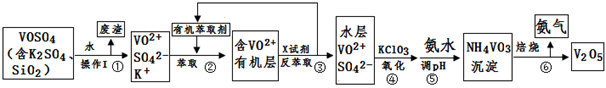
��ش��������⣺
��1����������÷����ijɷ��� ��д��ѧʽ��������I������ ��
��2������ڡ��۵ı仯���̿ɼ�Ϊ����ʽR��ʾVO2+��HA��ʾ�л���ȡ������
R2(SO4)n (ˮ��)+ 2nHA���л��㣩 2RAn���л��㣩 + nH2SO4 (ˮ��)
2RAn���л��㣩 + nH2SO4 (ˮ��)
������ȡʱ��������������ԭ���� ��
����X�Լ�Ϊ ��
��3���ݵ����ӷ���ʽΪ ��
��4��25��ʱ��ȡ����������������õ��������ʺ���ҺpH֮���ϵ���±���
| pH | 1.3 | 1.4 | 1.5 | 1.6 | 1.7 | 1.8 | 1.9 | 2.0 | 2.1 |
| ��������% | 88.1 | 94.8 | 96.5 | 98.0 | 98.8 | 98.8 | 96.4 | 93.1 | 89.3 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com