
����Cu2O���ھ��������Ĵ����ܶ��ܵ���ע���±�Ϊ��ȡCu2O�����ַ�����
| ������ | ��̿���ڸ��������»�ԭCuO |
| ������ | ��ⷨ����ӦΪ2Cu + H2O  Cu2O + H2���� Cu2O + H2���� |
| ������ | ���£�N2H4����ԭ����Cu(OH)2 |
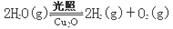 ��H >0
��H >0| ��� | �¶� | 0 | 10 | 20 | 30 | 40 | 50 |
| �� | T1 | 0.050 | 0.0492 | 0.0486 | 0.0482 | 0.0480 | 0.0480 |
| �� | T1 | 0.050 | 0.0488 | 0.0484 | 0.0480 | 0.0480 | 0.0480 |
| �� | T2 | 0.10 | 0.094 | 0.090 | 0.090 | 0.090 | 0.090 |
��1��ͭ��Cu
��2��-(a+b-2c)kJ/mol����2c �Ca-b��2�֣� ��3�֣�
��3��2Cu��2e����2OH��=Cu2O��H2O����3�֣�
��4��4Cu(OH)2 + N2H4 2Cu2O + N2�� + 6H2O����3�֣�
2Cu2O + N2�� + 6H2O����3�֣�
��5��C��3�֣�
���������������1����̿���ڸ��������»�ԭCuO�����²���������ͭ���ʶ�ʹCu2O���ʽ��͡�
��2�����ݸ�˹���ɵ������Ȼ�ѧ����ʽ����֪�Ȼ�ѧ����ʽ�Ĺ�ϵ����������֪�Ȼ�ѧ����ʽ�ֱ�Ϊ�٢ڢ��������Ȼ�ѧ����ʽ=��+��-2���ۣ�����2CuO(s)��C(s)= Cu2O(s)��CO(g)����H ="2c-a-b" kJ��mol-1
(3)��������������Ӧ��������ɵ�������ͭ�Ļ�ѧʽ�������������ĵ缫��Ӧ����ʽΪ2Cu��2e����2OH��=Cu2O��H2O
��4��N2H4��Cu(OH)2��Ӧ�������Cu2O ��N2���ˮ���ɣ����Ի�ѧ����ʽΪ4Cu(OH)2 + N2H4 2Cu2O + N2�� + 6H2O��
2Cu2O + N2�� + 6H2O��
��5��A�� ʵ��ڢ���ȣ�ʵ��۵�ˮ��������ʼŨ����ʵ��ڵ�2������ƽ��Ũ��ȴС��2����˵��T1��T2��ƽ�������ƶ���������ӦΪ���ȷ�Ӧ������T1��T2�������¶ȣ�T2 >T1,����B�����ݷ�Ӧ���ʵĶ���ʽ��ʵ���ǰ20 min��ƽ����Ӧ����v��H2O��=7��10-5 mol��L-1 min-1 ������v(O2)=3.5��10-5 mol��L-1 min-1 ������C��ʵ�����ʵ�����ȣ��ﵽ��ƽ��״̬��ͬ��������ʱ��̣���Ӧ���ʿ죬����ʵ��ڱ�ʵ������õĴ�����Ч�ʸߣ���ȷ����ѡC��
���㣺���鷴Ӧ������жϣ���˹���ɵ�Ӧ�ã���ѧ����ʽ����д����Ӧ���ʵļ��㼰ƽ���ƶ�ԭ����Ӧ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�о������ḻ��CO2��ȫ������Ϊ��̼Դ�������ǰӦ����㷺��̼Դ(ʯ�ͺ���Ȼ��)����������Ҷ���ݽߵ�Σ����ͬʱ�ֿɻ�����CO2�ۻ�������������ЧӦ��ʵ��CO2������ѭ����
��1��Ŀǰ��ҵ����һ�ַ�������CO2��H2��230�����������ת�����ɼ״�������ˮ��������ͼ��ʾ��ѹ������0.5 mol CO2��1.5 mol H2ת���ʴ�80%ʱ�������仯ʾ��ͼ��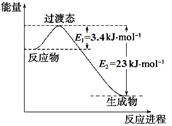
��д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
�����жϸ÷�Ӧ�ﵽ��ѧƽ��״̬�������� ��
a��������ѹǿ���� b��H2�������������
c��c(H2)=3c(CH3OH) d���������ܶȲ���
e��2��C��O���ѵ�ͬʱ��6��H��H���ѡ�
��2���˹���������ܹ�����̫���ܣ���CO2��H2O�Ʊ���ѧԭ�ϣ���ͼ��ͨ���˹���������Ʊ�HCOOHԭ����ʾ��ͼ������Ҫ��ش����⣺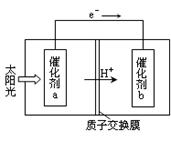
�ٸù����ǽ� ת��Ϊ ��(��������ѡ����ܡ���̫���ܡ�����ѧ�ܡ�)
�ڴ���b����ĵ缫��Ӧ����ʽΪ ��
��3��ij��������Ա�����ʹ�����������ͣ����ͻ�ѧʽ��C8H18��ʾ�����ȼ�ϵķ������Խ������CO2���ŷ����⡣�÷�����Ҫ���ô������CaH2����H2������������������������ʹ�ã�����ϵͳ�ֲ�������ȼ�ղ�����CO2����ϵͳ��Ӧ����ͼ��ʾ��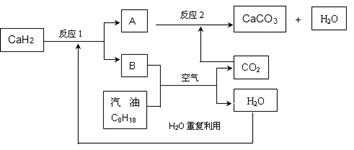
����������⣺
��д��CaH2�ĵ���ʽ ��
�ڷ�Ӧ1���������뻹ԭ�������ʵ���֮���ǣ� ��
�����ϵͳ��Ӧ��������ȫ����д����ϵͳ�ܷ�Ӧ�Ļ�ѧ����ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��֪��ѧ����������ʽ���ܿ����ת������д�±��Ŀհ�:
| ��ѧ��Ӧ����ʽ(����) | ����ת����ʽ |
| �� | �ɻ�ѧ��ת��Ϊ���� |
��Pb+PbO2+2H2SO4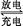 2PbSO4+2H2O 2PbSO4+2H2O | |
��CaCO3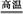 CaO+CO2�� CaO+CO2�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
(1)�״���һ����Ҫ�Ļ�����Ʒ�������ü״��������Ʊ���ȩ����ȩ����̬�״�ת����������ϵ��ͼ��ʾ��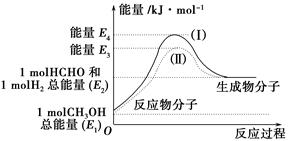
��Ӧ�����е�������ϵ
�ټ״�������ת��Ϊ��ȩ�ķ�Ӧ��________(����ȡ����ȡ�)��Ӧ��
�ڹ��̢�����̢�ķ�Ӧ���Ƿ���ͬ��____________ԭ����____________ ______________________________��
��д���״�������ת��Ϊ��ȩ���Ȼ�ѧ��Ӧ����ʽ______________ _____________________��
(2)��֪����CH3OH(g)��H2O(g)=CO2(g)��3H2(g)����H����49.0 kJ��mol��1
��CH3OH(g)��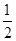 O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
O2(g)=CO2(g)��2H2(g)����H����192.9 kJ��mol��1
����˵����ȷ����________��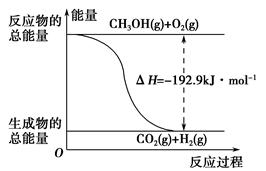
| A��CH3OHת���H2�Ĺ���һ��Ҫ�������� |
| B���ٷ�Ӧ�У���Ӧ�������������������������� |
C�����ݢ���֪��Ӧ��CH3OH(l)��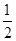 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 O2(g)=CO2(g)��2H2(g)�Ħ�H����192.9 kJ��mol��1 |
| D����Ӧ�ڵ������仯��ͼ��ʾ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
̼������ͬ����Ԫ�أ����������������Ź㷺����;��
��1�����������ȼ�ϵ�أ�������ʯī�缫����KOH��Һ�У��������ֱ�ͨ��CH4��O2�����ɼ���ȼ�ϵ�أ�ͨ��CH4��һ������缫��Ӧʽ�� ��
CH4����ԭNOX�������������������Ⱦ�������������β����Ⱦ���⣬��Ӧ���£�
CH4(g)+4NO2(g)=4NO(g)+CO2(g)+2H2O(g) ��H=��574kJ��mol��1
CH4(g)+4NO(g)=2N2(g)+CO2(g)+2H2O(g) ��H=��1160kJ��mol��1
��NO2�����黹ԭΪN2���Ȼ�ѧ����ʽΪ_____________________________________
��2����֪H2CO3 HCO3���� H�� Ka1��H2CO3��=4.45��10��7
HCO3���� H�� Ka1��H2CO3��=4.45��10��7
HCO3�� CO32����H�� Ka2(HCO3��)=5.61��10��11
CO32����H�� Ka2(HCO3��)=5.61��10��11
HA H����A�� Ka(HA)=2.95��10��8
H����A�� Ka(HA)=2.95��10��8
���������ϵ���ƽ�ⳣ����д������CO2ͨ�뵽NaA��Һ�е����ӷ���ʽ
___________________________��
��3�� ��T�¶�ʱ����1.0molCO2��3.0molH2����2L�ܱպ������У��ɷ�����Ӧ�ķ���ʽΪCO2 (g) + 3H2(g)  CH3OH(g) + 2H2O(g) ����ַ�Ӧ�ﵽƽ����������ڵ�ѹǿ����ʼѹǿ֮��Ϊa ��1����CO2ת ����Ϊ______����a=0.875ʱ���������´˷�Ӧ��ƽ�ⳣ��Ϊ_______________(�÷�����ʾ)��
CH3OH(g) + 2H2O(g) ����ַ�Ӧ�ﵽƽ����������ڵ�ѹǿ����ʼѹǿ֮��Ϊa ��1����CO2ת ����Ϊ______����a=0.875ʱ���������´˷�Ӧ��ƽ�ⳣ��Ϊ_______________(�÷�����ʾ)��
��4�������裨Si3N4����һ�������մɲ��ϣ�������ʯӢ(SiO2)�뽹̿�ڸ��µĵ������з�Ӧ���ɣ���֪�÷�Ӧ��ƽ�ⳣ������ʽK=��c(CO)��6/��c(N2)��2������֪CO��������Ϊv(CO)��6mol��L-1��min-1����N2��������Ϊv(N2)�� ���÷�Ӧ�Ļ�ѧ����ʽΪ________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��25 �桢101 kPa�������£�����1 mol H��H������436 kJ����������1 mol Cl��Cl������243 kJ�������γ�1 mol H��Cl���ų�431 kJ������H2��Cl2===2HCl�Ļ�ѧ��Ӧ������ͼ��ʾ��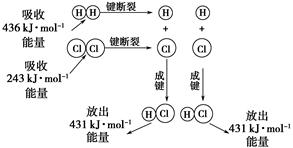
��ش������й����⣺
(1)��Ӧ��ϼ����յ�������Ϊ________��
(2)������ɼ��ų���������Ϊ________��
(3)�ж�H2��Cl2===2HCl��________(����ա��ų���)������
(4)��Ӧ���������________(�����������������)���������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�п�Ժ�����о����о�Ա���ʽ���������ͬ�к������Ա�������PM2.5��ѧ��ɼ���Դ�ļ��ڱ仯�о����֣�����PM2.5��6����Ҫ��Դ�����У�����β����ȼú�ֱ�ռ4%��18%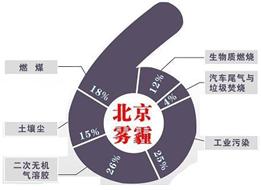
��1�����ھ�������β���ķ�ӦΪ��2NO(g)+2CO(g)
 2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
2CO2(g)+N2(g)����֪�÷�Ӧ��570Kʱ��ƽ�ⳣ��Ϊ1��1059������Ӧ���ʼ���������˵����ȷ���ǣ�________
| A��װ��β������װ�õ������ų��������в��ٺ���NO��CO |
| B�����β������Ч�ʵij��÷����������¶� |
| C������ѹǿ������ƽ�����ƣ���ʵ�ʲ����п�ͨ����ѹ�ķ�ʽ����侻��Ч�� |
| D�����β������Ч�ʵ����;����ʹ�ø�Ч���� |

 Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ
Ni(CO)4(g)������CO��Ӧ������������ж���Ϊ��ֹ�������ж�����ҵ�ϳ���SO2��ȥCO��������ΪS��CO2����֪��ط�Ӧ���̵������仯��ͼ��ʾ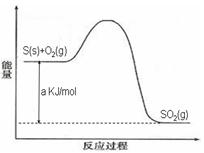
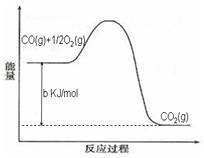
 2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
2N2(g)+3H2O(g)��H��0��Ϊ��ߵ��������ת���ʿɲ�ȡ�Ĵ�ʩ�ǣ�������һ�֣�____________________��
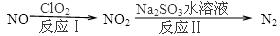
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ijʵ��С�������50 mL 1.0 mol/L�����50 mL 1.1 mol/L ����������Һ����ͼװ���н����кͷ�Ӧ���ڴ��ձ��ײ�������ĭ����(��ֽ��)��ʹ�����С�ձ���������ձ�������ƽ��Ȼ�����ڴ�С�ձ�֮����������ĭ����(��ֽ��)�����ձ�������ĭ���ϰ�(��Ӳֽ��)���ǰ壬�ڰ��м俪����С�ף�����ʹ�¶ȼƺͻ��β��������ͨ����ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȡ��Իش��������⣺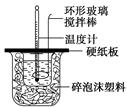
��1����ʵ�������Թ�����NaOH��ԭ��̲���˵��Ϊ��֤������ȫ���к͡����ʣ������ڷ�Ӧ������Ϊ�з�������������������ڷ�Ӧ�лӷ������õ��к���____________(�ƫ����ƫС�����䡱)��
��2�����к��Ȳⶨʵ���д�����ˮϴ���¶ȼ��ϵ�����IJ��裬���˲������裬���õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)��
��3�����õ�Ũ�ȵĴ�����NaOH��Һ��Ӧ�����õ��к��Ȼ�____________(�ƫ����ƫС�����䡱)����ԭ����_______________________________________________��
��4����ʵ��С����������ʵ�飬ÿ��ȡ��Һ��50 mL������¼��ԭʼ����(���±�)��
| ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�(t2)/�� | �²�(t2��t1)/�� | ||
| ���� | NaOH��Һ | ƽ��ֵ | |||
| 1 | 25.1 | 24.9 | 25.0 | 31.6 | 6.6 |
| 2 | 25.1 | 25.1 | 25.1 | 31.8 | 6.7 |
| 3 | 25.1 | 25.1 | 25.1 | 31.9 | 6.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����֪�ڳ��³�ѹ�£���2CH3OH(l)+3O2(g)�T2CO2(g)+4H2O(g) ��H=_1275��6kJ?mol-1
��H2O(l)�TH2O(g) ��H=+44��0kJ?mol-1д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ�� ��
�״�������ˮ������Ӧ������������Ӧ����ʽ���£�
CH3OH(g) + H2O(g)  CO2(g) + 3H2(g) ����H>0
CO2(g) + 3H2(g) ����H>0
��1��һ�������£������Ϊ2L�ĺ����ܱ������г���1molCH3OH(g)��3molH2O(g)��20s��û�������ѹǿ�Ƿ�Ӧǰ��1��2�������ü״���ʾ�÷�Ӧ������Ϊ���� �� ��
��2���ж����п��淴Ӧ�ﵽƽ��״̬�������ǣ�����ţ��� ����
��v��(CH3OH) = 3v��(H2)�� �ڻ��������ܶȲ��䡡 �ۻ�������ƽ����Է����������� ��CH3OH��H2O��CO2��H2��Ũ�ȶ����ٷ����仯 ��CO2��H2��Ũ��֮��Ϊ1:3
��3��ͼ��P�ǿ�����ƽ�л����Ļ������ر�K������ͬ�¶�ʱ����A�����г���1molCH3OH(g)��2molH2O(g)����B�����г���1��2molCH3OH(g) ��2��4molH2O(g)���������ֱ���������Ӧ�� ��֪��ʼʱ����A��B�������ΪaL����Ӧ�ﵽƽ��ʱ����B�����Ϊ1��5aL������B��CH3OHת����Ϊ�������� ����ά�������������䣬����Kһ��ʱ������´ﵽƽ�⣬����B�����Ϊ�������� L����ͨ��������������Բ��ƣ��Ҳ������¶ȵ�Ӱ�죩��
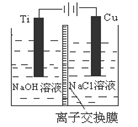
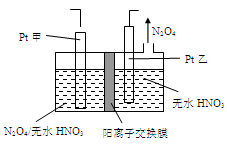
����ͼ�ס����ǵ绯ѧʵ��װ�á���ش��������⣺
��1���������о�ʢ��CuSO4��Һ
�ټ׳���ʯī���ϵĵ缫��ӦʽΪ____________________��
�������ʼʱ�ҳ�ʢ��200mL CuSO4��Һ�����һ��ʱ�����Һ��ɫ��dz����Ҫʹ��Һ�ָ������ǰ��״̬����Ҫ����Һ�м���0��8g CuO����������pHΪ ��������Һ����ı仯����
��2�����׳���ʢ�ű���NaCl��Һ����׳���ʯī���ϵĵ缫��ӦʽΪ__________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com