
��������ˮ�����г�����ɱ��������HClO��ɱ��������ClO��ǿ��25��ʱ����-��ˮ��ϵ�д�������ƽ���ϵ��
Cl2(g)  Cl2(aq) K1=10��1.2
Cl2(aq) K1=10��1.2
Cl2(aq)+ H2O  HClO + H+ +Cl�� K2=10��3.4
HClO + H+ +Cl�� K2=10��3.4
HClO  H+ + ClO�� Ka=?
H+ + ClO�� Ka=?
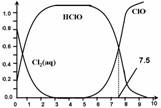 ����Cl2(aq)��HClO��ClO���ֱ�����������ռ����(��)��pH�仯�Ĺ�ϵ��ͼ��ʾ�����б�����ȷ����
����Cl2(aq)��HClO��ClO���ֱ�����������ռ����(��)��pH�仯�Ĺ�ϵ��ͼ��ʾ�����б�����ȷ����
A��Cl2(g)+ H2O  2H+ + ClO�� + Cl�� K=10��10.9
2H+ + ClO�� + Cl�� K=10��10.9
B�����ȴ���ˮ��ϵ�У�c(HClO) + c(ClO��) =c(H+)��c(OH��)
C�����ȴ�������ˮʱ��pH=7.5ʱɱ��Ч����pH=6.5ʱ��
D���ȴ�������ˮʱ�����ļ���ɱ��Ч�����ڶ�����
 ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ᡢ����������������������л��ﹲͬ���е�������(����)
A�����ܷ����ӳɷ�Ӧ B�����ܷ���ˮ�ⷴӦ
C�����ܸ�ϡH2SO4��Ӧ D�����ܸ�NaOH��Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
LiPF6������ӵ���й㷺Ӧ�õĵ���ʡ�ij������LiF��PCl5Ϊԭ�ϣ����·�Ӧ�Ʊ�LiPF6�����������£�
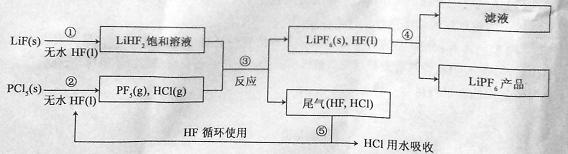
��֪��HCl�ķе��ǣ�85.0 �棬HF�ķе���19.5 �档
��1���ڢٲ���Ӧ����ˮHF�������� �� ����Ӧ�豸�����ò������ʵ�ԭ���� (�û�ѧ����ʽ��ʾ)����ˮHF�и�ʴ�ԺͶ��ԣ�������ȫ�ֲ���ʾ�������С�Ľ�HFմ��Ƥ���ϣ���������2%�� ��Һ��ϴ��
��2��������������ˮ�����½��У��ڢ۲���Ӧ��PCl5����ˮ�⣬�����Ϊ�����ᣬд��PCl5ˮ��Ļ�ѧ����ʽ�� ��
��3���ڢܲ�������õķ����� ���ڢݲ�����β����HF��HCl���õķ����� ��
��4��LiPF6��Ʒ��ͨ����������LiF��ȡ��Ʒwg�����Li�����ʵ���Ϊnmol�������Ʒ��LiPF6�����ʵ���Ϊ mol(�ú���w��n�Ĵ���ʽ��ʾ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
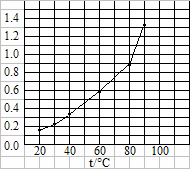
��������AgBrO3���ܽ�����¶ȱ仯������ͼ
��ʾ������˵���������
A�����������ܽ���Ƿ��ȹ���
B���¶�����ʱ�������ܽ��ٶȼӿ�
C��60��ʱ��������KspԼ����6��10-4
 D����������к��������������������ؽᾧ�����ᴿ
D����������к��������������������ؽᾧ�����ᴿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ����
A�������ԭˮ����ȵ��ˮ��������ܻ�����������
B��������ˮ(��NH4+��NH3)���û�ѧ��������绯ѧ����������
C��ij�ֹ�ѧ��⼼�����м��ߵ������ȣ��ɼ�����ϸ��(V=10��12L)�ڵ�����Ŀ����ӣ��ݴ˿�����ü�⼼���ܲ�����ϸ����Ũ��ԼΪ10��12��10��11mol ·L��1��Ŀ�����
D�������������Ӽ״��û��ȼ�ϵ���ֵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ȤС���Ա�����ϩΪ��Ҫԭ�ϣ���������·�ߺϳ�ҩ����³����
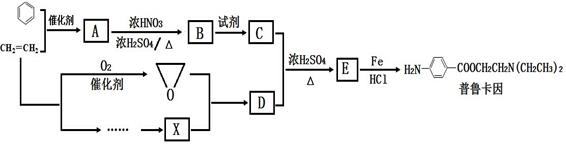
��֪��
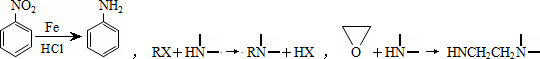
(1)������³��������˵����ȷ����________��
A������Ũ�����γ��� B���������������ӳɷ�Ӧ
C���ɷ���ˮ�ⷴӦ D�����γ�����
(2)д��������B�Ľṹ��ʽ________��
(3)д��B��C��Ӧ������Լ�________��
(4)д��C��D��E�Ļ�ѧ��Ӧ����ʽ________��
(5)д��ͬʱ��������������B������ͬ���칹��Ľṹ��ʽ________��
�ٷ����к����Ȼ�
��1H��NMR����ʾ�����к��б������ұ����������ֲ�ͬ��ѧ��������ԭ��
(6)ͨ��������ϩΪԭ���Ƶû����������X��Ӧ�ϳ�D�����û�ѧ��Ӧ����ʽ��ʾ����ϩΪԭ���Ʊ�X�ĺϳ�·��(���Լ���ѡ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�±���ÿ�����4��ѡ����������1��ѡ�������3��ѡ�����ڲ�ͬ�ķ��࣬�뽫������ѡ�����ż���ѡ���������±���
| ��� | ��ѡ�� | ����ѡ ����� | ��ѡ���� |
| (1) | A.S2�� B��I�� C��Fe��D��SO | ||
| (2) | A.HCl B��CO2 C��NH3 | ||
| (3) | A.����B����Һ C.������D���к� | ||
| (4) | A.KMnO4��B��Al2(SO4)3 C.KClO3�� D��K2HPO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����պŨ������ް��������Ͱ����Ĺܵ��Ƿ�©�� (����)
(2013���¿α�ȫ������7B)
(2)��SO2Ư��ֽ���Ͳ�ñ�� (����)
(2013���㶫���ۣ�11A)
(3)��������ϴ��¯�е�ˮ�� (����)
(2013���㶫���ۣ�11B)
(4)��Na2S������������ȥ��ˮ�е�Cu2����Hg2�� (����)
(2013���㶫���ۣ�11D)
(5)ʯӢֻ�������������ά (����)
(2013���������ۣ�6A)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���и������ǿ����ʡ�������ʡ��ǵ���ʵĹ��࣬��ȫ��ȷ���� (����)
|
| A | B | C | D |
| ǿ����� | Fe | NaCl | CaCO3 | HNO3 |
| ������� | CH3COOH | NH3 | H3PO4 | Ba(OH)2 |
| �ǵ���� | C12H22O11 (����) | BaSO4 | C2H5OH | H2O |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com