
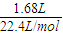 =0.075mol��
=0.075mol��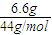 =0.15mol����n��C��=0.15mol��
=0.15mol����n��C��=0.15mol��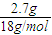 =0.15mol����n��H��=n��H2O��×2=0.3mol��
=0.15mol����n��H��=n��H2O��×2=0.3mol��

 �ο�����������100��ϵ�д�
�ο�����������100��ϵ�д� �Űٷֿ�ʱ����ϵ�д�
�Űٷֿ�ʱ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(1)����ȼ�ղ�����ˮ��������
(2)��ԭ�����ǵ�һ���壬ͨ�������ƶ����ķ���ʽ��
(3)��ԭ���������ֵ����ʵ���������Ļ�������ֻ��һ����������д�����ǵķ���ʽ��(ֻҪ��д��һ��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�л���A�����ĺ����������ͬ��ͬѹ�£�A������������ͬ����Ҵ�������2����1.38 g A��ȫȼ�պ�ȼ�ղ���ͨ����ʯ�ң���ʯ�ҵ���������3.06 g������ȼ�պ�IJ���ͨ��ŨH2SO4��ŨH2SO4����������1.08 g��ȡ4.6 g A�������Ľ����Ʒ�Ӧ���ڱ�״��������1.68 L H2��A��Na2CO3��Һ��ϲ���Ӧ����ͨ�������ƶϳ�A�Ľṹ��ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�Ҵ��ͱ����Ļ����7.6 g�����������Ľ����ƣ���Ӧ��Ϻ������H2�ڱ�״����Ϊ1.68 L�����������Ҵ�����������ʵ���֮��Ϊ( )
A.1:1 B.1:2 C.2:1 D.�����
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com