
����Ŀ��ijУ����С��Ϊ�ⶨij̼���ƺ�̼�����ƻ������̼���Ƶ������������ס�������ͬѧ�ֱ�������������ʵ�顣
��������ͬѧ�ó�����������������ͼ��ʾ��ʵ�����̽���ʵ�飺
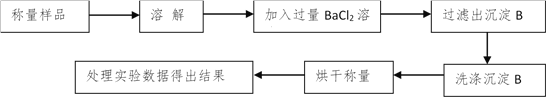
��1��ʵ��ʱ�����˲����У������ձ���©���⣬��Ҫ�õ��IJ�������Ϊ____________��
��2��ϴ�ӳ���B�IJ�����__________________________________________________��
��3����ʵ���в����Ʒ����Ϊm g����������Ϊn g����̼���Ƶ���������Ϊ________��

����������ͬѧ����Ҫʵ������ͼ���£�
������ͼ��ʾװ�ý���ʵ�飺
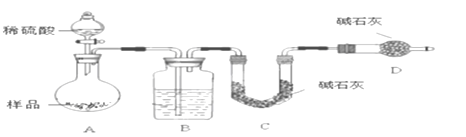
��4����ʵ����װ��Bʢ�ŵ�������_____________________����Һ©����___________����ܡ����ܡ������������ϡ�������ʵ�顣
��5����C��װ��ʯ�������վ���������塣Dװ�õ�������_________________��
��6���е�ͬѧ��ΪΪ�˼���ʵ�����ڷ�Ӧǰ��Ҫͨ��N2������ͼ����
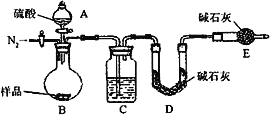
��Ӧ��ͨ��N2��Ŀ����_____________________��
���𰸡� ������ �ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2��3�� 106n/197m��100% Ũ���� ���� ���տ����е�ˮ������CO2����ȷ��ǰһ���������������������ȷ�� ��A��Bװ���в���CO2ȫ������Cװ�õļ�ʯ���У���С�������
����������������1�����˲��õ�������������̨��©�����ձ����������ȣ��������ڲ��������У�©�����ձ�����������
��2��ϴ�ӳ���Ҫע�ⲻ�ܳ�ϴ�������ò�����������ע������ˮ��ֱ���պ�û����������ˮ��©���ײ���Ȼ������ظ���������2~3����
��3������ΪBaCO3�����ݹ�ϵʽ��![]() ��̼���Ƶ���������Ϊ��
��̼���Ƶ���������Ϊ�� ![]() n g��m g��100%=
n g��m g��100%=![]() ��100%��
��100%��
����������ʵ�����̡�װ��ͼ��ʵ��Ŀ�Ŀɵã�A�в���CO2��H2O��B����ˮ����װ�ã�C�м�ʯ������A������CO2��D�м�ʯ����������������������ͨ������C���������صķ������A������CO2���������Ӷ�̼���Ƶ�����������
��4��B����ˮ����װ�ã���Bʢ�ŵ�Һ����Ũ�����Һ©���в������������ϡ�������ʵ�飬��Ϊ�����ǻӷ����ᣬ������ʹC��������HCl����������ƫ��
��5��D��ʢ�м�ʯ�ң������տ����е�ˮ������CO2���Ա�֤C��������������ȷ����
��6��ʵ�����ʱ��װ�����Դ���CO2���壬��Ӧ��ͨ��N2�ɽ�A��Bװ���в�����CO2ȫ������Cװ�õļ�ʯ���У���С���������


 ���100��1�ž�ϵ�д�
���100��1�ž�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��һ�ֿ����ú����ˮ��ȡKI��ʵ���������£�
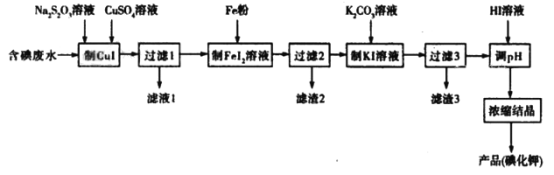
�ش��������⣺
(1)�����ˮ�е�Ĵ�����ʽһ����I2��I-�������Ƿ�I2��I-�ķ�������CCl4��ȡ��۲���ȡҺΪ��ɫ��֤������I2��������ȡ��ȡˮ���������Թ��У�_____________��
(2)����CuI��ʱ��I2����ԭΪCuI��ͬʱ��S4O62�����ɡ�
�ٸ÷�Ӧ�����ӷ���ʽΪ_________________________________________________��
����֪������Ӧ��ƽ�ⳣ��Ϊ1.78��1026��ʵ���������CuSO4����2������Ŀ����_________________��
(3)����FeI2��Һ���Ļ�ѧ����ʽΪ____________________________________��������3���ijɷ���________________(�ѧʽ)��
(4)��HI������ҺpHԼΪ6����Ŀ����_____________________________________��
(5)ȷ��ȡ��Ʒ0.1000g����50mLˮ�������������Ტ���������ָʾ����Ȼ����0.025mol��L-1AgNO3��Һ�ζ������յ�ʱ����AgNO3��Һ22.00mL�����Ʒ��KI����������Ϊ____________(������λ��Ч����)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A ��һ�ַ�����Ϊ28����̬��������AΪ��Ҫԭ�Ϻϳ�һ�־��й���ζ������E����ϳ�·����ͼ1��ʾ�� 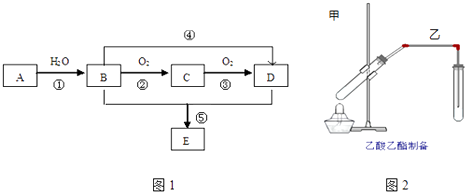
��ش��������⣺
��1��B�����й����ŵ�����Ϊ ��
��2��Bͨ�����������ɵõ�D��Ҳ��ͨ����Ӧ��ֱ������ΪD������Ҫ����������Լ�Ϊ������һ�֣���
��3��д�����з�Ӧ�Ļ�ѧ����ʽ��
������Ӧ���ͣ� ��
�� ��
��4����Ӧ�ݿɰ��½��У�
��ʵ�鲽�衿
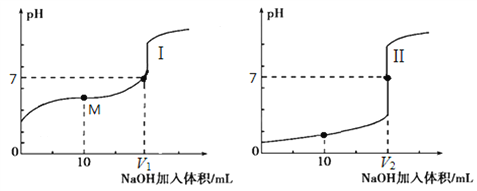
��ͼ2���Թܼ����ȼ���2mL 95%��B������ҡ���»�������2mLŨ���ᣬ���ҡ�ȣ���ȴ���ټ���2mL D���ò�������ֽ�����Թ̶ܹ�������̨�ϣ����Թ����м���5ml ����̼������Һ����ͼ2���Ӻ�װ�ã��þƾ��ƶ��Թܼ�С�����3��5min���ô����ȣ����۲쵽���Թ�������������ʱֹͣʵ�飮�Իش�
��ʵ������
a���Թ����й۲쵽�������� ��
b���Թܼ��з�Ӧ�Ļ�ѧ����ʽΪ �� ��Ӧ����Ϊ ��
������̽����
��ʵ�鷴Ӧ��ʼʱ��С����ȵ�ԭ���� ��
���������Ͽ�֪�����������ķе�Ϊ77�棻�Ҵ��ķе�Ϊ78.5�棻����ķе�Ϊ117.9�棩
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ʵ���������������
A. ��ʪ���pH��ֽ��ϡ��Һ��pH���ⶨֵƫС
B. ������ƿ������Һ������ʱ���ӿ̶��ߣ�������ҺŨ��ƫС
C. �ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫС
D. �ⶨ�кͷ�Ӧ�ķ�Ӧ��ʱ��������������У������¶�ֵƫС
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У���������� ��
A. �����ӵ������ķ���ΪNA�������ֵΪ6.02��1023 mol��1
B. NA�����ӵ����ʵ�����1 mol
C. ��ͬ��ͬѹ�£���ͬ������κ����嵥�������ķ�����Ŀ��ͬ
D. H2SO4��Ħ��������M����98
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
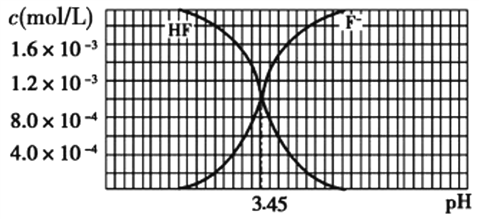
����Ŀ��������0.1 molL-1 NaOH��Һ�ֱ�ζ������Ϊ20.00 mL��Ũ�Ⱦ�Ϊ0.1 molL-1������ʹ�����Һ���õ��ζ���������ҺpH�����NaOH��Һ������仯�������ζ����ߡ�
�ٵζ������������______________���I��������
�ڵζ���ʼǰ��������Һ����ˮ�������c(H+)������_____________________��
��V1��V2�Ĺ�ϵ��V1 _____ V2�����������������������
������֪25��ʱ��2.0��103 mol/L�����ˮ��Һ�У�������ҺpH(��������仯)���õ�c(HF)��c(F)����ҺpH�ı仯��ϵ��ͼ��ʾ��
��1��25��ʱ��HF�ĵ���ƽ�ⳣ��Ka=_____________��
��2����֪HF(aq)![]() H+(aq)+F(aq) ��H=10.4 kJ/mol���ֽ�������0.1 mol/L HF��Һ������30�������������ʺ��ܼ��Ļӷ��������и����������__________��
H+(aq)+F(aq) ��H=10.4 kJ/mol���ֽ�������0.1 mol/L HF��Һ������30�������������ʺ��ܼ��Ļӷ��������и����������__________��
a��Ka b��Kw c��n(H+) d�� 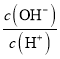
��3��25��ʱ����20 mL 0.1 mol/L������м���V mL 0.1 mol/L NaOH��Һ����û����Һ��pH�仯������ͼ��ʾ������˵����ȷ����__________________��
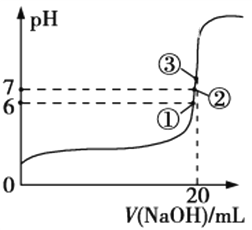
a��pH=3��HF��Һ��pH=11��NaF��Һ�У���ˮ�������c(H+)���
b���ٵ�ʱpH=6����ʱ��Һ�У�c(F)c(Na+)=9.9��107 mol/L
c���ڵ�ʱ����Һ�е�c(F)=c(Na+)
d���۵�ʱV=20 mL����ʱ��Һ��c(F)��c(Na+)=0.1 mol/L
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������ӻ����ͬһ��Һ�У���Ϊ��ٽ�ˮ������ܴ���������ǣ� ��
A.Na+��Al3+��Cl-��AlO2-B.Ba2+��NH4+��Cl-��OH-
C.H3O+��Ca2+��PO43-��Cl-D.Na+��NH4+��Cl-��CO32-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵����ȷ����
A. ������ͬ��ԭ������SO2��CO������֮��Ϊ7:8
B. �����ʵ���Ũ�ȵ�����������е�c(H+)���
C. �����ʵ����ļ���-CH3�����ǻ���-OH���������������
D. ���µ�ѹ�£�SO2������CO2������ܶ�֮�ȵ���11��16
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й����ʵķ�����ȷ����
���������� | һԪ�� | ǿ����� | ��ɢϵ | |
A | Mn2O7 | ���� | ���������� | �ƺ�ˮ |
B | NO2 | ������ | ���� | ��ɫ���� |
C | SiO2 | ʯ̿�� | �������� | �ơ��� |
D | SO2 | ���� | �廯�� | ��ˮ����� |
A. AB. BC. CD. D
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com