
 �ǻ���ʯ��һ����Ҫ�����������ϣ��䳣�õ��Ʊ����������֣�
�ǻ���ʯ��һ����Ҫ�����������ϣ��䳣�õ��Ʊ����������֣����� ��1���ٴ��غ�ĽǶȿ�֪��5molCa��NO3��2��3mol��NH4��2HPO4��4molNH3•H2O��Ӧ����1molCa5��PO4��3OH��10molNH4NO3��3molH2O��
�ڴ��غ�ĽǶȿ�֪��5molCa��OH��2��3molH3PO4��Ӧ����1molCa5��PO4��3OH��9molH2O��
��2���뷽��A��ȣ�����B���ŵ��Ǹ���ƷΪˮ��û����������Ʒ�����ռ�
��3����ӦҺ�ֲ����Թ�����CaHPO4������
��4������pH��������7.39��7.41���ܽ��ԽСԽ�ȶ���
��� �⣺��1�����غ�ĽǶȿ�֪��5molCa��NO3��2��3mol��NH4��2HPO4��4molNH3•H2O��Ӧ����1molCa5��PO4��3OH��10molNH4NO3��3molH2O����Ӧ�Ļ�ѧ����ʽΪ��5Ca��NO3��2+3��NH4��2HPO4+4NH3•H2O=Ca5��PO4��3OH��+10NH4NO3+3H2O
�ʴ�Ϊ��10NH4NO3��3H2O��
�ڴ��غ�ĽǶȿ�֪��5molCa��OH��2��3molH3PO4��Ӧ����1molCa5��PO4��3OH��9molH2O��
��Ӧ�Ļ�ѧ����ʽΪ5Ca��OH��2+3H3PO4=Ca5��PO4��3OH��+9H2O��
�ʴ�Ϊ��Ca5��PO4��3OH��+9H2O��
��2������A���и�����NH4NO3��ԭ�������ʲ��ߣ��뷽��A��ȣ�����B���ŵ��Ǹ���ƷΪˮ��û����������Ʒ�����ռ�
�ʴ�Ϊ��Ψһ������Ϊˮ�����ռ�
��3������B�У����H3PO4��Һ�μӹ��죬�ᵼ�¾ֲ����Թ�������CaHPO4���ƵõIJ��ﲻ����
�ʴ�Ϊ����ӦҺ�ֲ����Թ�����CaHPO4������
��4������pH��������7.39��7.41����������Ca5��PO4��3OH�ܽ����С�����Ը���Ҫ������ʽΪCa5��PO4��3OH���ʴ�Ϊ��Ca5��PO4��3OH��
���� ���⿼��ʵ���Ʊ��������漰��ѧ����ʽ��д���Է���������ķ������ۡ�ͼ������ȣ��ǶԻ���֪ʶ���ۺ�Ӧ�ã���Ҫѧ���߱���ʵ�Ļ������Ѷ��еȣ�


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��������ѹǿ | B�� | ���������ܶ� | ||
| C�� | C��D�����ʵ����ı�ֵ | D�� | ����������ʵ��� | ||
| E�� | �����ƽ��Ħ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
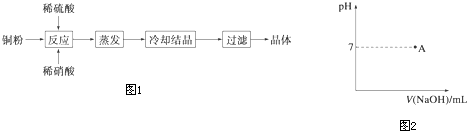
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
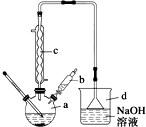 �屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
�屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�| �� | �� | �屽 | |
| �ܶ�/g•cm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����������������
��������Ϊ��������| A�� | 3��3-����-2-�һ�-���� | B�� | 3��3-����-4-�һ����� | ||
| C�� | 2��3��3-�������� | D�� | 3��3��4-�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 44g | B�� | 22g | C�� | 44g/mol | D�� | 22g/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| ���� | Pb2+ | Ca2+ | Fe3+ | Mn2+ | Cl- |
| ����ǰŨ��/��mg•L-1�� | 0.100 | 29.8 | 0.120 | 0.087 | 51.9 |
| ������Ũ��/��mg•L-1�� | 0.004 | 22.6 | 0.040 | 0.053 | 49.8 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com