

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����A��D���飬ÿ����������Ӧ������������Ӧ����ͬһ�����ӷ���ʽ��ʾ���ǣ�������
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.NaOH��Һ��ϡH2SO4��KOH��Һ��ϡ����
B.Fe2O3��ϡHNO3��Fe2O3�������
C.NaHSO4��Һ��NaHCO3��Һ��NaHCO3��Һ��ϡ����
D.NaAlO2����ˮ��NaAlO2��Һ�е�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ������ʡ�Ӱ�����߿�ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
��.2009��ī���硢�����ȵس��ּ���H1N1�������飬����ȫ����Ĺ�ע�����������������ɼ���H1N1��������ġ������������ƺ�Ԥ�������Ƽ�ʹ����ʿ������ҩ��˾�����ġ���ơ����Һ�Ӣ�������ء�ʷ�˹�˾����������ʽҩ�����ָ��塱����ҩ�
��ƺ��ָ���Ľṹ��ʽ����ͼ��ʾ��


��ش��������⣺
��1���ָ���ķ���ʽ��____ ��
��2���ϳɴ�Ƶ���Ҫԭ��ç����(�ṹ��ʽΪ 
 )�������ҹ�ʢ���İ˽������С�����ç�������ʵ�����������˵����ȷ���ǣ� ��
)�������ҹ�ʢ���İ˽������С�����ç�������ʵ�����������˵����ȷ���ǣ� ��
A. 1 molç�����������Ľ����Ʒ�Ӧ������4mol H2��
B. 1 molç��������������������Һ��Ӧ������������������4 mol��
C. 1 molç������������̼������Һ��Ӧ�����ɶ�����̼����1 mol��
D. 1 molç������������̼��������Һ��Ӧ�����ɶ�����̼����1 mol
��3������ÿ���и����������ʣ����Ƕ����÷�Һ©���������
A������������ˮ���ƾ���ˮ��ֲ���ͺ�ˮ
B�����Ȼ�̼��ˮ���屽��ˮ����������ˮ
C�����ͺ�ˮ�������ˮ��������Ҵ�
D�����ͺ�ˮ������ˮ���Ҷ�����ˮ
��.��ͼ��A��B��C��D��E��Ϊ�л��������֪��C�ܸ�NaHCO3��Һ������Ӧ��C��D����Է���������ȣ���EΪ��֧���Ļ������ش��������⣺
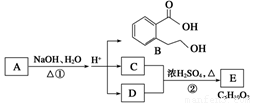
��1��C�����й����ŵ�������������������������B���ܷ����ķ�Ӧ����������(����ĸ���)��
a���ӳɷ�Ӧ ��b��ȡ����Ӧ ��c����ȥ��Ӧ��
d��������Ӧ e��ˮ�ⷴӦ�� f��������Ӧ
��2����Ӧ�ڵĻ�ѧ����ʽ��___________________________����3�� A�Ľṹ��ʽ��_________________��
��4��ͬʱ������������������B��ͬ���칹�����Ŀ��______����
a���м��ȡ�������ṹ�� b.���ڷǷ������� c.����FeCl3��Һ������ɫ��Ӧ
д����������һ��ͬ���칹��Ľṹ��ʽ__________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�߶���ѧ����ĩ���Ի�ѧ ���ͣ�ѡ����
����A~D���飬ÿ����������Ӧ������������Ӧ����ͬһ�����ӷ���ʽ��ʾ����
|
|
��I�� |
��II�� |
|
A |
����SO2ͨ��Ba(OH)2��Һ |
����SO2ͨ������Ba(OH)2��Һ |
|
B |
����Ũ��ˮ����Al2(SO4)3��Һ |
����Al2(SO4)3��Һ����Ũ��ˮ |
|
C |
0.1mol Cl2ͨ�뺬0.2mol FeBr2����Һ |
0.3 molCl2ͨ��1L 0.2 mol•L��1 FeBr2��Һ�� |
|
D |
����BaCl2��Һ������Na2SO4��Һ���� |
����Ba(OH)2��Һ�����MgSO4��Һ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com