
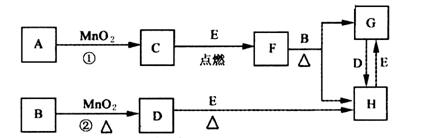
(1)д����ѧʽ��B______________��E_____________��
(2)ָ��MnO2����ط�Ӧ�е����ã���Ӧ������_________������Ӧ������__________����
(3)���F��B������Ӧ��ѧ����ʽ_______________________��
(4)����Ӧ�����ڼ��������½��У�A��________________������Ӧ�����ڳ��������½��У�A��__________________�����������������µõ�������C���ʣ���Ӧ��ת�Ƶĵ�����֮��Ϊ___________________��
 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2006�����������ۺ�ģ�⻯ѧ���֡�(��һ��) ���ͣ�022
| |||||||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ����һ�и�һ��ѧ����ĩ���Ի�ѧ�Ծ� ���ͣ������
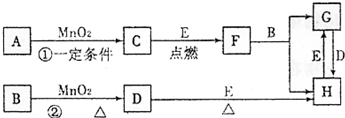
��12�֣���ͼ�漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�C��D��E��FΪ���ʣ�����������Ϊ����������������Ϊ���������Һ�����Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ��
��1��д����ѧʽ��A ��E ��
��2��ָ��MnO2����ط�Ӧ�е����ã���Ӧ������ ������Ӧ������ ����
��3����Ӧ�۵Ļ�ѧ����ʽΪ ��
��4��G������B��NaNO3��ϡ��Һ��Ϸ�Ӧ����H����д���˷�Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com