
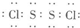
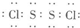
| a |
| 64 |
| a |
| 64 |
| 46Q |
| b |
| 46Q |
| b |
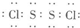 £¬¹¹³ÉĪ¢Į£ĪŖ·Ö×Ó£¬ŌņĪŖ·Ö×Ó¾§Ģ壬¹Ź“š°øĪŖ£ŗ
£¬¹¹³ÉĪ¢Į£ĪŖ·Ö×Ó£¬ŌņĪŖ·Ö×Ó¾§Ģ壬¹Ź“š°øĪŖ£ŗ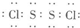 £»·Ö×Ó¾§Ģ壻
£»·Ö×Ó¾§Ģ壻| ag |
| 64g/mol |
| a |
| 64 |
| a |
| 64 |
| 46Q |
| b |
| 46Q |
| b |
| 46Q |
| b |


 Ģ½¾æÓė¹®¹ĢŗÓÄĻæĘѧ¼¼Źõ³ö°ęÉēĻµĮŠ“š°ø
Ģ½¾æÓė¹®¹ĢŗÓÄĻæĘѧ¼¼Źõ³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā
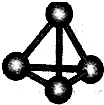 ĻÖÓŠA”¢B”¢C”¢D”¢E”¢FĮłÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬DÓėEµÄĒā»ÆĪļ·Ö×Ó¹¹ŠĶ¶¼ŹĒVŠĶ£®A”¢BµÄ×īĶā²ćµē×ÓŹżÖ®ŗĶÓėCµÄ×īĶā²ćµē×ÓŹżĻąµČ£¬AÄÜ·Ö±šÓėB”¢C”¢DŠĪ³Éµē×Ó×ÜŹżĻąµČµÄ·Ö×Ó£¬ĒŅAÓėDæÉŠĪ³ÉµÄ»ÆŗĻĪļ£¬³£ĪĀĻĀ¾łĪŖŅŗĢ¬£®
ĻÖÓŠA”¢B”¢C”¢D”¢E”¢FĮłÖÖ¶ĢÖÜĘŚŌŖĖŲ£¬ĖüĆĒµÄŌ×ÓŠņŹżŅĄ“ĪŌö“ó£¬DÓėEµÄĒā»ÆĪļ·Ö×Ó¹¹ŠĶ¶¼ŹĒVŠĶ£®A”¢BµÄ×īĶā²ćµē×ÓŹżÖ®ŗĶÓėCµÄ×īĶā²ćµē×ÓŹżĻąµČ£¬AÄÜ·Ö±šÓėB”¢C”¢DŠĪ³Éµē×Ó×ÜŹżĻąµČµÄ·Ö×Ó£¬ĒŅAÓėDæÉŠĪ³ÉµÄ»ÆŗĻĪļ£¬³£ĪĀĻĀ¾łĪŖŅŗĢ¬£®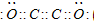
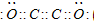
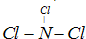
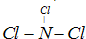
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ








²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ




²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| DµÄµ„ÖŹ |
| DµÄµ„ÖŹ |
| H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com