
����ݽ̲��ϵ�ʵ�顰����������Ӧ��������������⡣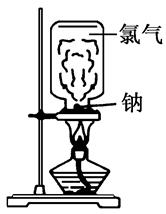
��1��ȡһ���̶���Ľ����ƣ���ȥ�����㣩������ֽ����������ú�ͣ�����ʯ�����ϣ��þƾ����ȡ������۳���״ʱ����ʢ�������ļ���ƿѸ�ٵ������Ƶ��Ϸ���������ѧ��֪ʶ������ʵ�������Щȱ�㣿
��____________________________________________________��
��____________________________________________________��
��________________����������2����
��2��ijͬѧ�������ϴ��ڵ�ȱ��Ľ�ʵ��װ�ã���ͼ��ʾ��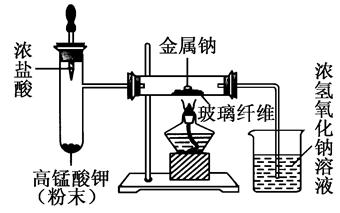
ʵ�鲽�裺
a��ȡ�̶�������ƣ�����ֽ���ɱ����ú�ͣ���ȥ�����㣬���벣�����У���ͼʾ��װ��������
b�������μ�Ũ���ᣬ�������ҷ�Ӧ����������
c�����������г�������ɫ����ʱ���ټ����ƣ����ۻ���ȼ�ա�
�ٹ۲쵽��ʵ�������У����μ�Ũ������Թ��в���________ɫ���壻�ƾ���ȼ�գ������________ɫ����________���ɣ���Ӧ�����ܱ��Ϲ۲쵽��________���ɡ�
�ڸĽ����ʵ���ŵ㣺a.________��b.________��c.______�����ٻش�2������
��д��Na��Cl2��ȼ�յĻ�ѧ����ʽ________________�����õ���ʽ��ʾ�����γɹ���________________��
 �������¿��ÿ�ʱ��ҵϵ�д�
�������¿��ÿ�ʱ��ҵϵ�д� Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
Ӣ�żƻ�ͬ����ʱ��Чѵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
��һС�������Ͷ�뵽ʢ��100ml AlCl3��MgCl2�Ļ����Һ���ձ��У������ձ��������ݲ�������������л������ձ����а�ɫ�����������������ȶ���١���Ӧ��Ϻ��ռ�����״��������13.44Lͬʱ�õ�21.4g���������������ڹ�����NaOH��Һ�У����ֳ���������15.6g����ԭ�����Һ��Mg2+��Al3+��Cl-�����ʵ���Ũ�ȡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ�ˡ�̽�������仯����������Ի�ԭ�ԡ���
��1��ʵ��ǰͬѧԤ�⣺Fe2+�϶����л�ԭ�����������ԣ�����Ϊ��Ԥ��������ǣ�__
��2����ͬѧ����ʵ��֤������Ԥ�⣮ʵ�����ṩ�������Լ���3����H2O2��Һ��п����ͭƬ��0��1mol��L -lFeCl2��Һ��KSCN��Һ��������ˮ��
�����ƻ���0��1 mol��L-l FeCl2��Һ�е���������ˮ��̽��Fe2+�Ļ�ԭ�ԣ���Ԥ�ƿ��ܷ�����Ӧ�����ӷ���ʽΪ
��ʵ���У���ͬѧ��������̫���ԣ���ʦ���������Dz���ĺ���̫�ͣ��������ͨ������Fe2+��Ӧ�IJ���Ĵ����Ի�ȡ֤�ݣ�����Ϊ��ѡ_____����С�����õĻ��Һ�У���ͨ����Һ����___ɫ������֤����ͬѧ�Ĺ۵��ʵ�鷽��������ȷ�ģ�
�۶���֤��Fe2+���������ԣ���ͬѧ��Ϊ�������ʶ����л�ԭ�ԣ����ֱ�ͭƬ��п��Ͷ��FeCl2��Һ�У����п����С���ɴ�˵�����ֽ����Ļ�ԭ����ǿ������˳��Ϊ________��
��3����ͬѧ����H2O2����Ԫ���ԣ�1��(�м��)����������ʣ�H2O2��FeCl2�ķ�Ӧʱ��Fe2+������������
������Ϊ��ͬѧ����������⣺��Fe2+�ڷ�Ӧ�б��ֳ�������Ӧת����______(�������ţ���ͬ)����Fe2+�ڷ�Ӧ�б��ֳ���ԭ��Ӧת����_____��
��ʵ����Fe2+��ԭ�Խ�ǿ��ʵ���ҵ�FeCl2��Һ�������������ʣ����ӵķ����ǣ� ����ط�Ӧ�����ӷ���ʽ��_____ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ʵ���Ҳⶨ̼������̼�����ƵĻ�����У�̼���Ƶ���������[�÷���w(Na2CO3)��ʾ]����ȡ�˻����5.lg������ˮ�У����250mL��Һ��
a��(10��)����һ����������w(Na2CO3)���û�ѧ��Ӧ��HCO3����CO32����ȫת��Ϊ��������ȡ�������������ɴ˼���������w (Na2CO3)��
��1����ȡ100 mL���ƺõ���Һ���ձ��У��μ�����������������Һ��HCO3����CO32����ȫת��Ϊ������Ӧѡ���Լ���___________ �����ţ���
| A��CaCl2 | B��MgSO4 | C����NaCI | D��Ba(OH)2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������������������ͭ���Ǻ�ɫ��ĩ�����������ϡ�ijУһ��ѧʵ��С��ͨ��ʵ����̽��һ��ɫ��ĩ��Fe2O3��Cu2O����ߵĻ���̽���������£�
�������ϣ�Cu2O��һ�ּ������������ϡ��������Cu��CuSO4���ڿ����м�������CuO��
�������
����1����ɫ��ĩ��Fe2O3
����2����ɫ��ĩ��Cu2O
����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ����
���̽��ʵ��
ȡ������ĩ��������ϡ�����У���������Һ���ٵμ�KSCN�Լ���
��1��������1��������ʵ��������_____________________________________________��
��2�����μ�KSCN�Լ�����Һ�����ɫ����֤��ԭ�����ĩ��һ����������������������Ϊ����˵��������________�������������(����д����Ӧ����ʽ)____________
________________________________________________________________________��
��3���������ĩ��ȫ�ܽ�������ڣ��μ�KSCN�Լ�ʱ��Һ�����ɫ����֤��ԭ�����ĩ��________��д��������Ӧ�����ӷ���ʽ________________________________��
̽������
��ʵ�������ȷ����ɫ��ĩΪFe2O3��Cu2O�Ļ���
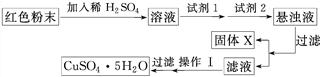
��4��ʵ��С�����ü��ȷ��ⶨCu2O������������ȡa g�����ĩ�ڿ����г�ּ��ȣ����������ٱ仯ʱ����������Ϊb g(b��a)����������Cu2O����������Ϊ________��
��5��ʵ��С�������øú�ɫ��ĩ��ȡ�ϴ����ĵ���(CuSO4��5H2O)�����������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2����Fe2����Fe3���ֱ����ɳ�����pH���£�
| ���� | Cu(OH)2 | Fe(OH)2 | Fe(OH)3 |
| ��ʼ����pH | 6.0 | 7.5 | 1.4 |
| ������ȫpH | 13 | 14 | 3.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
Ϊ��̽��SO2��Na2O2�ķ�Ӧ�Ƿ�������CO2��Na2O2�ķ�Ӧ����ͬѧ�������ͼ��ʾ��ʵ��װ�ã��ش��������⣺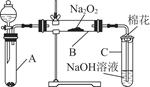
��1���ƿ������������ǵ�ľ������C�Թܿڣ�δ��ľ����ȼ����ͬѧ�����ΪSO2��Na2O2�ķ�Ӧ��ͬ��CO2���밴��ͬѧ�Ĺ۵�д����Ӧ�Ļ�ѧ����ʽ ��
��2����ͬѧ��Ϊ���۷�Ӧԭ����Σ����ն���O2��������ͬѧ�������� ��������ͬѧ�Ĺ۵㣬��װ�������ĸĽ���
��
��3������Na2O2��ȫ��Ӧ����Ӧ��Bװ���й�������������ǣ���Na2SO3����Na2SO4����Na2SO3��Na2SO4��
�����ʵ�鷽�����飬д��ʵ�鲽���Լ�Ԥ������ͽ��ۣ�����±���
��ѡ�Լ���2 mol��L��1 HCl��Һ��1 mol��L��1 HNO3��Һ��1 mol��L��1 BaCl��Һ��1 mol��L��1 Ba��NO3��2��Һ��0.01 mol��L��1 KMnO4������Һ��
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����1��ȡB�е�����������Ʒ���Թ��У��μ���������ˮ���ܽ⣬Ȼ��ȡ��������Һ�ֱ����ڢ��Թ��� | ������ȫ�ܽ� |
| ����2�������Թ��м��� ���ٵμ� | �� |
| ��֤���������к�Na2SO4 | |
| ����3�������Թ��� | |
| | �� �� |
| ��֤������������Na2SO3���� | |
| | |
| ��˵����������û��Na2SO3�� | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
һ��ⶨ��Ʒ�гɷֺ�����ʵ��Ӧ�ظ�2��3�Ρ�Ϊ�˲ⶨij�������ƹ����л��е�̼���Ƶ������������ס��ҡ�����λͬѧ�ֱ����������ʵ�鷽����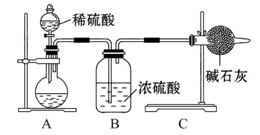
��ͬѧ�ķ�����ͼ��ʾ��
��1����μ���Aװ�õ������ԣ�_____________________________________________��
��2����ͬѧ�ظ�����������ʵ�飬�õ�̼���Ƶ��������������ݴ��ڽϴ��ƫ�����Ϊ��������������ƫ�͵�ԭ����_______������ţ���
A��װ����ԭ�п����еĶ�����̼����Ҳ����ʯ������
B��װ��������е�ˮ�����Ͷ�����̼����ʯ������
C����Ӧ��ɺ�װ���еĶ�����̼û��ȫ������ʯ������
D������ϡ����������㡢��Ӧ�����
��3��Ϊ���ü�ʵ����������ȷ��������ʵ�鲽�趼��ȷ�������£�����Ϊͼ�е�ʵ��װ��Ӧ����θĽ���______________��
����ͬѧ�ķ����ǣ���ͼ�����ṩ��װ����ѡ��ʵ��װ�ã������ͬѧʵ��װ���е�B��C��ͨ���ⶨ�ų��Ķ�����̼������������Ƕ�����̼����ˮ�������㡣
ѡ�����װ�õ�����˳��Ϊ_______��
��ͬѧ�ķ����ǣ���ȡ��Ʒm g�����ܽ⣬��������Ȼ�����Һ�����ˡ�ϴ�ӡ���ɡ��������ù���n g��
��1������100 mL 0��10 mol/L BaCl2��Һ��ʵ��������IJ����������ձ�������������ͷ�ιܡ���Ͳ���_______�����������ƣ���
��2���������̼���Ƶ���������Ϊ����m��n��ʾ��_______��
��3��Ca2+��Ba2+������ʹ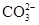 ������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
������ȫ���ܷ�ʹ���Ȼ�����Һ�����Ȼ�����Һ��_______ ����ܡ�����ԭ���ǣ�_____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
ijͬѧ�������������������̼���Ʒ�ĩ�п��ܺ��������Ȼ��ƺ����������е�һ�ֻ��������ʡ�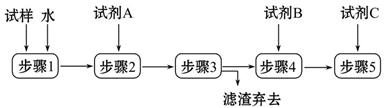
(1)����1���貣��������______������3�IJ���������______��
(2)�Լ���̼���Ʒ�ĩ�п��ܺ��е���������������裺
����1��ֻ�����Ȼ��ƣ�
����2��ֻ����___________��
����3���Ȼ��ƺ��������ƶ����С�
(3)���ʵ�鷽��������ʵ�顣
��ѡ�����Լ����Ȼ�����Һ�����ᱵ��Һ����̪��Һ��ϡ���ᡢϡ���ᡢϡ���ᡢ��������Һ���ش��������⣺
�ټ�������Լ�A��������______��
������:
| ʵ�鲽�� | Ԥ������ͽ��� |
| ����4: | |
| ����5: | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����Mg��CO2�ķ�Ӧ�Ʋ⣬NaҲ����CO2��ȼ�գ��ҹ���������ΪC��Na2O��Na2CO3�е����ֻ����֡�ij��ȤС��������װ�ÿ�չ�����ε�ʵ��̽����
��ʵ��I������CO2��Na��Ӧ����������Ϊ��
�ٰ�ͼ����װ�ã�����װ�������ԣ�������װ���м����Լ���
�ڴ�װ��a�ϻ���һ��ʱ�䣻
�۵�ȼd���ƾ��ƣ�ʹCO2��Na��ַ�Ӧ��ֹͣ���ȣ�����ͨ����ʹ˫ͨ����ȴ��
�ش��������⣺
��1��װ��a���õ��IJ����������Թܺ� ��
��2��װ��b��װ����Լ������������������������� ��
��3��װ��c������������������������������ ��
��4��������У����۲쵽������������������ ʱ�����ܽ��벽��ۡ�
��ʵ���̽����Ӧ���P��Ӧ������ȡ��Ӧ��˫ͨ���й�������29.2 g��������ʵ�飺
����ϸ�۲���壬�����к�ɫ������
�ڽ���������������ˮ�����ˡ�ϴ�ӣ��õ�1.8 g�����������
�۽���Һ��ˮϡ�����250 mL����Һ��
��ȡ�����۵���Һ���ȼ�����BaCl2��Һ���۲쵽��ɫ����,���ù�������Ϊ3.94g���ټӼ��η�̪��Һ����Һ��죻
��ȡ25.00 mL�۵���Һ���μӼ�����Ϊָʾ������3.0 mol��L-1����ζ��������������Ϊ20.00 mL��
�ش��������⣺
��5������жϢ��еĵζ��յ�����������������������
��6����Ӧ��װ��d�еĹ�������Ϊ���������������������� ��ͨ�����������29.2 g���������У�����ֵ��������Ƕ���?����������������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com