
£Ø18·Ö£©Ä³»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēĶ¼£©£¬ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©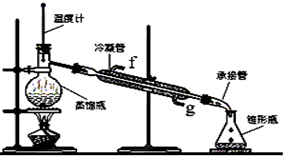
£Ø1£©Öʱø“ÖĘ·
½«12.5 mL»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ¼ÓČėl mLÅØĮņĖį£¬Ņ”ŌČŗó·ÅČėĖé“Éʬ£¬»ŗĀż¼ÓČČÖĮ·“Ó¦ĶźČ«£¬ŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·”£
¢ŁAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ____________£¬µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠµÄ×÷ÓĆŹĒ_________”£
¢ŚŹŌ¹ÜCÖĆÓŚ±łĖ®Ō”ÖŠµÄÄæµÄŹĒ______________________________”£
£Ø2£©Öʱø¾«Ę·
¢Ł»·¼ŗĻ©“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹµČ”£¼ÓČė±„ŗĶŹ³ŃĪĖ®£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ć£¬»·¼ŗĻ©ŌŚ_______²ć(ĢīÉĻ»ņĻĀ)£¬·ÖŅŗŗóÓĆ_______ (ĢīČė±ąŗÅ)Ļ“µÓ”£
A£®KMnO4ČÜŅŗ B£®Ļ”H2SO4 C£® Na2CO3ČÜŅŗ
¢ŚŌŁ½«»·¼ŗĻ©°“ĻĀĶ¼×°ÖĆÕōĮó£¬ĄäČ“Ė®“Ó_________æŚ½ųČė£¬ÄæµÄŹĒ__________________”£
¢ŪŹÕ¼Æ²śĘ·Ź±£¬æŲÖʵÄĪĀ¶ČÓ¦ŌŚ ×óÓŅ”£ŅŌĻĀĒų·Ö»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·µÄ·½·Ø£¬ŗĻĄķµÄŹĒ_________”£
A ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ B ÓĆ½šŹōÄĘ C ²ā¶Ø·Šµć
£Ø18·Ö£©£Ø1£©¢Ł·Ą±©·Š ĄäÄż ¢Ś·ĄÖ¹»·¼ŗĻ©»Ó·¢
£Ø2£©¢ŁÉĻ C ¢Ś g ĄäČ“Ė®ÓėĘųĢåŠĪ³ÉÄęĮ÷ ¢Ū 83”ę C
½āĪöŹŌĢā·ÖĪö£ŗæ¼²éŹµŃé×ŪŗĻ·ÖĪöÄÜĮ¦£¬£Ø1£©¢ŁAÖŠ¼ÓČėĖé“ÉʬµÄ×÷ÓĆŹĒ·ĄÖ¹ŅŗĢå¾Ö²æŹÜČČ²»¾ł·¢Éś±©·ŠĢŚĻÖĻó£»×¢Ņā¹Ū²ģµ¼¹ÜBµÄ³¤¶Č£¬æÉĶʵĆBµ¼¹Ü³żĮĖµ¼ĘųĶā»¹¾ßÓŠĄäÄżµÄ×÷ÓĆ”£
¢ŚÓÉĢāŅā£¬½«»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ»ŗĀżÓĆĖ®Ō”¼ÓČČ·¢Éś·“Ó¦£¬»·¼ŗĻ©»Ó·¢³öĄ“£¬¾¹żĄäÄżŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·£¬ÓÉ»·¼ŗĻ©µÄČŪ”¢·ŠµćæÉÖŖĄäĖ®Ō”µÄ×÷ÓĆŹĒ·ĄÖ¹»·¼ŗĻ©µÄ»Ó·¢£¬ŅŌµĆµ½øü¶ąµÄ²śĘ·£»£Ø2£©¢ŁŹµŃéÄæµÄŹĒĢį“æ»·¼ŗĻ©£¬ÓÉÓŚ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹÄÜČÜÓŚĖ®ÖŠ£¬¶ų»·¼ŗĻ©ÄŃČÜÓŚĖ®£¬»·¼ŗĻ©ĆܶȊ”ÓŚĖ®µÄĆÜ¶Č£¬ĖłŅŌ»·¼ŗĻ©ŌŚÉĻ²ć£¬±„ŗĶŹ³ŃĪĖ®ŌŚĻĀ²ć£»·ÖŅŗŗóÓŠĖįŠŌ²ŠĮō£¬ĖłŅŌÓĆNa2CO3ČÜŅŗ¼īŠŌČÜŅŗĻ“µÓ£»¢Ś½«Ģį“æŗóµÄ»·¼ŗĻ©ÕōĮóŹ±£¬ĄäČ“Ė®“ÓgæŚ½ųČė£¬ÄæµÄŹĒČĆĄäČ“Ė®ÓėĘųĢåŠĪ³ÉÄęĮ÷£¬Ź¹øßĪĀĘųĢåµĆµ½³ä·ÖµÄĄäÄż£»¢ŪŹÕ¼Æ»·¼ŗĻ©²śĘ·Ź±£¬æŲÖʵÄĪĀ¶ČÓ¦ŌŚĘä·Šµć83”ę×óÓŅ£¬ÕāŃłµĆµ½µÄĮó·Ö²ÅŹĒ»·¼ŗĻ©£»Ēų·Ö»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·µÄ·½·Ø£¬²»ÄÜÓĆAĖįŠŌøßĆĢĖį¼ŲČÜŅŗ£¬ŅņĪŖ¶¼ÓŠÓÉ×ĻÉ«±äĒ³µÄĻÖĻó£»Ņ²²»ÄÜÓĆB½šŹōÄĘ£¬ŅņĪŖ»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·ÖŠ¶¼æÉÄÜŗ¬ÓŠĖ®£¬ĖłŅŌĻÖĻóĻąĶ¬¶ų²»ÄÜĒų·Ö£¬Ń”ŌńC²ā¶Ø·Šµć½ĻŗĻĄķ£¬ŅņĪŖ“æ¾»ĪļÓŠ¹Ģ¶ØµÄ·Šµć£¬¶ų»ģŗĻĪļƻӊ¹Ģ¶Ø·Šµć”£
æ¼µć£ŗæ¼²éÓŠ»śĪļµÄÖĘČ”ŗĶĢį“棬»¹æ¼²éĮĖŹµŃé²Ł×÷ŗĶ·ÖĪöÄÜĮ¦
µćĘĄ£ŗ±¾ĢāŹĒŠÅĻ¢øųÓčĢāŠĶ£¬½įŗĻŹŌĢāŠÅĻ¢ŗĶŹµŃ黳“”ÖŖŹ¶£¬Įé»ī·ÖĪö¾ĶæÉµĆ³öŗĻĄķ“š°ø”£×¢ŅāĘ½Ź±Ń§Ļ°Ź±£¬Ąķ½āŗĆŹÆÓĶµÄ·ÖĮó”¢ŅŅĖįŅŅõ„µÄÖĘČ”µČ»ł“”ŹµŃ飬²ÅÄÜŌŚ“ĖĄą×ŪŗĻĢāÖŠÓĪČŠÓŠÓą”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ


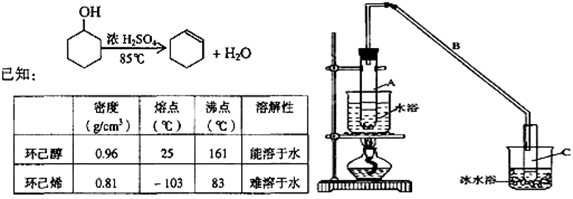
| ĆÜ¶Č£Øg/cm3£© | ČŪµć£Ø”ę£© | ·Šµć£Ø”ę£© | ČܽāŠŌ | »·¼ŗ“¼ | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® | »·¼ŗĻ© | 0.81 | -103 | 83 | ÄŃČÜÓŚĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŌĶĮĄķ½ā


| ĆÜ¶Č£Øg/cm3£© | ČŪµć£Ø”ę£© | ·Šµć£Ø”ę£© | ČܽāŠŌ | »·¼ŗ“¼ | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® | »·¼ŗĻ© | 0.81 | -103 | 83 | ÄŃČÜÓŚĖ® |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ij»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēĶ¼¢ń)ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©”£

ŅŃÖŖ£ŗ
| ĆÜ¶Č£Øg”¤cm-3) | ČŪµć£Ø”ę)·Š | µć£Ø”ę) | ČܽāŠŌ | |
| »·¼ŗ“¼ | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® |
| »·¼ŗĻ© | 0.81 | -103 | 83 | ÄŃČÜÓŚĖ® |

Ķ¼¢ń
(1)Öʱø“ÖĘ·
½«12.5 mL»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ¼ÓČė1 mLÅØĮņĖį£¬Ņ”ŌČŗó·ÅČėĖé“Éʬ£¬»ŗĀż¼ÓČČÖĮ·“Ó¦ĶźČ«£¬ŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·”£
¢ŁAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ_____________£¬µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠµÄ×÷ÓĆŹĒ_______________”£
¢ŚŹŌ¹ÜCÖĆÓŚ±łĖ®Ō”ÖŠµÄÄæµÄŹĒ_________________________________________________”£
£Ø2£©Öʱø¾«Ę·
¢Ł»·¼ŗĻ©“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹµČ”£ÖŠŗĶ±„ŗĶŹ³ŃĪĖ®£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ć£¬»·¼ŗĻ©ŌŚ_______________²ć£ØĢī”°ÉĻ”±»ņ”°ĻĀ”±£©£¬·ÖŅŗŗóÓĆ_______________£ØĢīČė±ąŗÅ£©Ļ“µÓ”£
a.KMnO4ČÜŅŗ
b.Ļ”H2SO4
c.Na2CO3ČÜŅŗ

Ķ¼¢ņ
¢ŚŌŁ½«»·¼ŗĻ©°“Ķ¼¢ņ×°ÖĆÕōĮó£¬ĄäČ“Ė®“Ó____________æŚ½ųČė”£ÕōĮóŹ±ŅŖ¼ÓČėÉśŹÆ»Ņ£¬ÄæµÄŹĒ____________”£
¢ŪŹÕ¼Æ²śĘ·Ź±£¬æŲÖʵÄĪĀ¶ČÓ¦ŌŚ____________×óÓŅ£¬ŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ棬æÉÄܵÄŌŅņŹĒ________________________”£
a.ÕōĮ󏱓Ó70 ”ęæŖŹ¼ŹÕ¼Æ²śĘ·
b.»·¼ŗ“¼Źµ¼ŹÓĆĮæ¶ąĮĖ
c.Öʱø“ÖĘ·Ź±»·¼ŗ“¼Ėę²śĘ·Ņ»ĘšÕō³ö
£Ø3£©ŅŌĻĀĒų·Ö»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·µÄ·½·Ø£¬ŗĻĄķµÄŹĒ____________”£
a.ÓĆøßĆĢĖį¼ŲĖįŠŌČÜŅŗ b.ÓĆ½šŹōÄĘ c.²ā¶Ø·Šµć
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğŗÓ±±Ź”ĢĘɽŅ»ÖŠø߶žĻĀŃ§ĘŚĘŚÖŠæ¼ŹŌ»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
(11·Ö)ij»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ(ČēĶ¼)£¬ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©”£
ŅŃÖŖ£ŗ

| | ĆܶČ(g/cm3) | ČŪµć(”ę) | ·Šµć(”ę) | ČܽāŠŌ |
| »·ŅŃ“¼ | 0.96 | 25 | 161 | ÄÜČÜÓŚĖ® |
| »·ŅŃĻ© | 0.81 | £103 | 83 | ÄŃČÜÓŚĖ® |

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2011-2012ѧğ¹ć¶«Ź”Ć·ÖŻŹŠŌųĻÜč÷֊ѧø߶ž5ŌĀŌĀæ¼»ÆѧŹŌ¾ķ£Ø“ų½āĪö£© ĢāŠĶ£ŗŹµŃéĢā
£Ø20·Ö£©Ä³»ÆѧŠ”×é²ÉÓĆĄąĖĘÖĘŅŅĖįŅŅõ„µÄ×°ÖĆ£ØČēĶ¼£©£¬ŅŌ»·¼ŗ“¼Öʱø»·¼ŗĻ©
£Ø1£©Öʱø“ÖĘ·
½«12.5mL»·¼ŗ“¼¼ÓČėŹŌ¹ÜAÖŠ£¬ŌŁ¼ÓČėlmLÅØĮņĖį£¬Ņ”ŌČŗó·ÅČėĖé“Éʬ£¬»ŗĀż¼ÓČČÖĮ·“Ó¦ĶźČ«£¬ŌŚŹŌ¹ÜCÄŚµĆµ½»·¼ŗĻ©“ÖĘ·”£
¢ŁAÖŠĖé“ÉʬµÄ×÷ÓĆŹĒ____________£¬µ¼¹ÜB³żĮĖµ¼ĘųĶā»¹¾ßÓŠµÄ×÷ÓĆŹĒ____ ”£
¢ŚŹŌ¹ÜCÖĆÓŚ±łĖ®Ō”ÖŠµÄÄæµÄŹĒ_____________________________”£
£Ø2)Öʱø¾«Ę·
¢Ł»·¼ŗĻ©“ÖĘ·ÖŠŗ¬ÓŠ»·¼ŗ“¼ŗĶÉŁĮæĖįŠŌŌÓÖŹµČ”£¼ÓČė±„ŗĶŹ³ŃĪĖ®£¬Õńµ“”¢¾²ÖĆ”¢·Ö²ć£¬»·¼ŗĻ©ŌŚ______²ć(ĢīÉĻ»ņĻĀ)£¬·ÖŅŗŗóÓĆ_________ (ĢīČė±ąŗÅ)Ļ“µÓ”£
A£®KMnO4ČÜŅŗ B£®Ļ”H2SO4 C£®Na2CO3ČÜŅŗ
¢ŚŌŁ½«»·¼ŗĻ©°“ĻĀĶ¼×°ÖĆÕōĮó£¬ĄäČ“Ė®“Ó_________æŚ½ųČė”£ÕōĮóŹ±ŅŖ¼ÓČėÉśŹÆ»Ņ£¬ÄæµÄŹĒ______________ ____”£
¢ŪŹÕ¼Æ²śĘ·Ź±£¬æŲÖʵÄĪĀ¶ČÓ¦ŌŚ_________×óÓŅ£¬ŹµŃéÖʵƵĻ·¼ŗĻ©¾«Ę·ÖŹĮæµĶÓŚĄķĀŪ²śĮ棬æÉÄܵÄŌŅņŹĒ____________________
A£®ÕōĮ󏱓Ó70”ęæŖŹ¼ŹÕ¼Æ²śĘ·
B£®»·¼ŗ“¼Źµ¼ŹÓĆĮæ¶ąĮĖ
C£®Öʱø“ÖĘ·Ź±»·¼ŗ“¼Ėę²śĘ·Ņ»ĘšÕō³ö
£Ø3£©ŅŌĻĀĒų·Ö»·¼ŗĻ©¾«Ę·ŗĶ“ÖĘ·µÄ·½·Ø£¬ŗĻĄķµÄŹĒ_________”£
A£®ÓĆĖįŠŌøßĆĢĖį¼ŲČÜŅŗ B£®ÓĆ½šŹōÄĘ C£®²ā¶Ø·Šµć
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com