
��������ʮ�����ʣ���Һ̬HCl ��NaHCO3 ��NaCl��Һ ��CO2 �����Ǿ��� ��Ba(OH)2 �ߺ��ɫ�������������� ��NH3��H2O ����� ��Al2(SO4)3
��1������ʮ��������������������ˮ��Һ�пɷ�����Ӧ�����ӷ���ʽΪ��H����OH��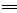 H2O���÷�Ӧ�Ļ�ѧ����ʽΪ ��
H2O���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��2������ˮ�еĵ��뷽��ʽΪ ��
��3��θҺ�к������ᣬθ�������˳���θ�����ĵĸо���������ˮ������������С�մ�NaHCO3����������θ����࣬��д���䷴Ӧ�����ӷ���ʽ�� ���������ͬʱ��θ����Ϊ��θ�ڴ��ף����ܷ���С�մ�ʱ����ú�Al(OH)3��θҩ����θ��ƽ��������θ�ᷴӦ�����ӷ���ʽ��
��4����Ba(OH)2��Һ����μ���ϡ���ᣬ������������⣺
��д����Ӧ�����ӷ���ʽ ��
27����1��Ba��OH��2+2HCl=BaCl2+2H2O����2�� Al2��SO4��3=2Al3++3SO42-�� ��3��HCO3-+H+=H2O+CO2����Al��OH��3+3H+=Al3++3H2O����4��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O
��������
�����������1�������������ڿ�����ǿ����Ȼ������ڿ�����ǿ�ᣬ���߷�Ӧ�Ļ�ѧ����ʽΪ��Ba��OH��2+2HCl=BaCl2+2H2O�����ӷ���ʽ���Ա�ʾΪ��H++OH-�TH2O����Ϊ��Ba��OH��2+2HCl=BaCl2+2H2O����2����������ǿ����ʣ���ˮ����ȫ���룬���뷽��ʽΪ��Al2��SO4��3=2Al3++3SO42-����Ϊ��Al2��SO4��3=2Al3++3SO42-����3��С�մ������ᷴӦ�����ӷ���ʽΪ��HCO3-+H+=H2O+CO2��������������������Ӧ�����ӷ���ʽΪ��Al��OH��3+3H+=Al3++3H2O����Ϊ��HCO3-+H+=H2O+CO2����Al��OH��3+3H+=Al3++3H2O����4������Ba��OH��2��Һ����μ���ϡ���ᣬ��Ӧ�������ᱵ��ˮ�������ӷ�ӦΪ��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O����Ϊ��Ba2++2OH-+SO42-+2H+�TBaSO4��+2H2O��
���㣺�������ӷ���ʽ�����뷽��ʽ����д��


 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�������ʡ�������и�����һ�β��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
һ���¶��£���ӦN2(g)��O2(g)==2NO(g)���ܱ������н��У����д�ʩ���ı仯ѧ��Ӧ���ʵ���
A��������ϵ�¶� B�����ݣ�����N2
C�����ݣ�����He D����ѹ������He
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���ֽ���������ĩ15 g�������������ᷴӦʱ���ɱ�״����11.2 L������������������Ľ����������
A��Zn��Fe B��Zn��Ag C��Al��Cu D��Mg��Al
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
���������У���ֻ�������ԡ�ֻ�л�ԭ�ԡ��������������л�ԭ�Ե�˳�����е�һ����
A��O2��K��HCl B��Cl2��Al��H2
C��NO2��Na��Br2 D��O2��SO2��H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ�������������ȷ����
A����100ml��Ͳ��ȡ8.5ml��ˮ
B��Ϊ�˲�����˷ѣ�ʵ��ʣ���ҩƷ�Ż�ԭƿ
C�����ձ����ڵ���ʯ��������Ȧ�ϼ���
D����ȼ�ŵľƾ���ȥ��ȼ��һ�ƾ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ˫Ѽɽ�и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��CH4��CO��ɵĻ�����壬�ڱ�״���µ��ܶ�Ϊ���硤�̣���������������CH4��CO��������Ϊ
A��1��1 B��7��8 C��2��3 D��1��2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�������ʡ˫Ѽɽ�и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵����ȷ����
A������ԭ��ʧ����Խ�࣬�仹ԭ��Խǿ
B��CH4��Ħ������Ϊ16g
C��10 mL��������Ϊ98%��H2SO4����ˮϡ����100 mL��H2SO4����������Ϊ9.8%
D����������ʱ������ܵ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���ຣʡ�����и�����ѧ�ڵ�һ���¿����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
��15�֣�Ϊ��̽��H2O2��H2SO3��Br2�����Ե����ǿ�����������ʵ�飨�г���������ȥ������ش��������⣺

��1������A������_________����������___________��
��2��������B�μ�Һ�岢����Ҫ������c��ԭ����____________________________��
��3��ʵ���¼���£��벹ȫ�հף���
���� | ʵ����� | ʵ������ | ʵ����� |
�� | ����a����μ���H2SO3��Һ������ | ________________ | __________________________ |
�� | �����������Һ����μ���H2O2��Һ | �տ�ʼ��Һ��ɫ�����Ա仯�������μӣ���Һ��Ϊ�Ȼ�ɫ | __________________________ |
��4��������У���ʼʱ��ɫ�����Ա仯��ԭ���ǣ�д��һ����_______________________��
������з�Ӧ�����ӷ���ʽ_________________________________________________��
���������Ҫ��Ӧ�����ӷ���ʽ_____________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015������ʡ�߶���ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�����£���20��00 mL 1��000 mol��L��1��ˮ�е���1��000 mol��L��1���ᣬ��ҺpH���¶��������������仯������ͼ��ʾ�������й�˵���������

A��a��d�������Һ��ˮ�����ӻ�Kw(a)>Kw(d)
B�����˰�ˮϡ�ͣ���Һ�ĵ�����������
C��c��ʱ�����������V(HCl)<20 mL
D�������£�a��İ�ˮ���볣��Ϊ mol��L��1
mol��L��1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com