
ij��ɫ����Һ�п��ܴ�������Ag����Mg2����Cu2���еļ������ӡ�
(1)�����κ�ʵ��Ϳ��Կ϶�ԭ��Һ�в����ڵ�������________��
(2)ȡ����ԭ��Һ�������ϡ���ᣬ�а�ɫ�������ɣ��ټ������ϡ���ᣬ��ɫ��������ʧ��˵��ԭ��Һ�п϶��е�������________���йص����ӷ���ʽΪ__________________ ________��
________��
(3)ȡ(2)����Һ�������NaOH��Һ�����ְ�ɫ������˵��ԭ��Һ�п϶����ڵ�������________��
(4)ԭ��Һ�п��ܴ������ڵ�������������A��D�е�(�����)________��
A��Cl�� B��NO
C��CO D��OH��
D��OH��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
N2O5��һ����������������һ���¶��¿ɷ������з�Ӧ��
2N2O5(g)  4NO2(g)��O2(g)����H>0
4NO2(g)��O2(g)����H>0
T1�¶��µIJ���ʵ������Ϊ��
| t/s | 0 | 500 | 1 000 | 1 500 |
| c(N2O5)/mol/ L | 5.00 | 3.52 | 2.50 | 2.50 |
����˵������ȷ����(����)
A��500 s��N2O5�ֽ�����Ϊ2.96��10��3 mol/(L��s)
B��T1�¶��µ�ƽ�ⳣ��ΪK1��125,1 000 sʱת����Ϊ50%
C��������������ʱ��T2�¶��·�Ӧ��1 000 sʱ���N2O5(g)Ũ��Ϊ2.98 mol/L����T1<T2
D��T1�¶��µ�ƽ�ⳣ��ΪK1��T3�¶��µ�ƽ�ⳣ��ΪK3����K1>K3����T1>T3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���һԪ��HA����Һ��KOH��Һ�������ϣ���������仯����ʵ���������±���
| ʵ���� | ��ʼŨ��/(mol•L��1) | ��Ӧ����Һ��pH | |
| c(HA) | c(KOH) | ||
| �� | 0.1 | 0.1 | 9 |
| �� | x | 0.2 | 7 |
�����ж�����ȷ���� �� ��
A��ʵ��ٷ�Ӧ�����Һ�У�c(K+) > c(A��) > c(OH��) > c(H+)
B��ʵ��ٷ�Ӧ�����Һ�У�c(OH��) = c(K+) �� c(A��) =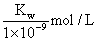
C��ʵ��ڷ�Ӧ�����Һ�У�c(A��) + c(HA) > 0.1mol/L
D��ʵ��ڷ�Ӧ�����Һ�У�c(K+) = c(A��) > c(OH��) = c(H+)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��Һ�д��ڽ϶��OH����K+��CO32��������Һ�л����ܴ������ڵ���( )
A��H+ B��Ca2+ C��SO42�� D��NH4+
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����и�����������Һ�У�һ�������������������
A��1.0 mol��L��1��Na2CO3��Һ��
Fe2����H����Cl����SO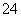
B�����ȳʺ�ɫ����Һ��Na����K����SiO ��NO
��NO
C����ˮ�������c(H��)��2.0��10��10����Һ�У�
Ba2����K����Cl����HCO
D��������Al3������Һ�У�Cu2����Na����SO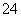 ��NO
��NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͬһ��Һ�У�����NH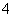 ��I����Ba2����Cl�������ӣ������Һ���ܵ�pH�ͽ�����
��I����Ba2����Cl�������ӣ������Һ���ܵ�pH�ͽ�����
A��pH��1��ϡ����Ϊ���� B��pH��3������Ϊ����
C��pH��8������������ҺΪ���� D��pH��12����ˮΪ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ˮ��ҺX��ֻ��������K����Mg2����Al3����AlO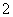 ��SiO
��SiO ��CO
��CO ��SO
��SO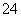 �е����������ӡ�ijͬѧ�Ը���Һ����������ʵ�飺
�е����������ӡ�ijͬѧ�Ը���Һ����������ʵ�飺
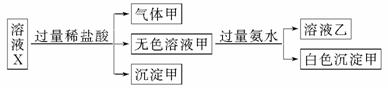
�����ж���ȷ����
A�������һ���Ǵ�����
B���������ǹ������þ�Ļ����
C��K����AlO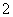 ��SiO
��SiO һ����������ҺX��
һ����������ҺX��
D��CO ��SO
��SO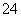 һ������������ҺX��
һ������������ҺX��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
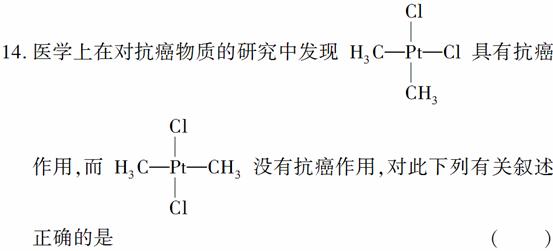
A������Ϊͬ���칹�壬������Ptԭ��Ϊ���ĵ�������ṹ
B������Ϊͬ���칹�壬������Ptԭ��Ϊ���ĵ�ƽ��ṹ
C������Ϊͬһ���ʣ�������Ptԭ��Ϊ���ĵ�������ṹ
D������Ϊͬһ���ʣ�������Ptԭ��Ϊ���ĵ�ƽ��ṹ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������У�ֻ�������Ӽ��������й��ۼ�����
A��HCl B��KOH C��CaCl2 D��CO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com