
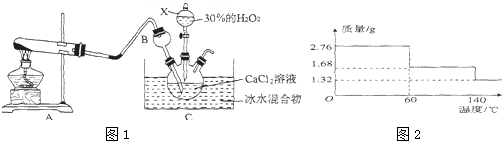
���� ��1������X��������֪Ϊ��Һ©����������©�����¿ڲ���ˮ�У��������������������м��飻
��2��������������ˮ����ֹ������
��3��ʵ���������Ȼ�狀��������ƹ�����ȡ������
��4������O�棬Һ�����ᣬ��Ӧ���ѣ��¶ȹ��ߣ���������ֽ����ʼӿ죻
CaCl2��H2O2��NH3��H2O��Ӧ����CaO2•8H2O��NH4Cl��
��5����140��ʱ��ȫ��ˮ���������Ȳ��ֽ⣬�����Ʒ������������ᾧˮ�������������ʵ������Ӷ��ó���Ʒ��CaO2•8H2O���ʵ������ٸ���60��ʱ��������������ʧȥ�ᾧˮ�����������ʵ��������������CaO2•xH2O�е�x��
�ڸ��ݢټ��������Ʒ��CaO2�����ʵ���������m=nM��������������ټ������Ʒ��CaO2•8H2O�Ĵ��ȣ�
��� �⣺��1������X��������֪XΪ��Һ©����������©�����¿ڲ���ˮ���������Թ��ڹܿ������ݲ��������ֺ��γ�һ���ȶ���ˮ����
�ʴ�Ϊ����Һ©����������©�����¿ڲ���ˮ���������Թ��ڹܿ������ݲ��������ֺ��γ�һ���ȶ���ˮ����
��2��A�в����İ�����������C�У�Bװ�ÿ��Է�������
�ʴ�Ϊ����������
��3��ʵ���������Ȼ�狀��������ƹ�����ȡ��������Ӧ����ʽΪ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
�ʴ�Ϊ��Ca��OH��2+2NH4Cl$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��4�����ڵ���0�棬Һ�����ᣬ��Ӧ���ѣ����¶Ƚϸߣ���������ֽ����ʼӿ죬������ȡCaO2•8H2Oһ����0�桫5��ĵ����½��У�
����CaO2•8H20�Ļ�ѧ����ʽΪ��CaCl2+H2O2+2NH3+8H2O=2NH4Cl+CaO2•8H2O��
�ʴ�Ϊ������0�棬Һ�����ᣬ��Ӧ���ѣ��¶Ƚϸߣ���������ֽ����ʼӿ죻CaCl2+H2O2+2NH3+8H2O=2NH4Cl+CaO2•8H2O��
��5����140��ʱ��ȫ��ˮ���������Ȳ��ֽ⣬����Ʒ��CaO2•8H2O���еĽᾧˮ��������Ϊ��2.76g-1.32g=1.44g���ᾧˮ�����ʵ���Ϊ��$\frac{1.44g}{18g/mol}$=0.08mol��ԭ��Ʒ�к���CaO2•8H2O�����ʵ���Ϊ��$\frac{0.08mol}{8}$=0.01mol��
60��ʱ���������Ϊ1.68g��ʧȥ�ᾧˮ������Ϊ��2.76g-1.68g=1.08g��ʧȥ�ᾧˮ�����ʵ���Ϊ��$\frac{1.08g}{18g/mol}$=0.06mol����60��ʱCaO2•xH2O��x=$\frac{0.08mol-0.6mol}{0.01mol}$=2��
�ʴ�Ϊ��2��
�ڸ��ݢٿ�֪CaO2•8H2O�����ʵ���Ϊ0.01mol����CaO2�����ʵ���Ҳ��0.01mol��������Ϊ��72g/mol��0.01mol=0.72g����Ʒ��CaO2�Ĵ���Ϊ��$\frac{0.72g}{2.76g}$��100%��26.09%��
�ʴ�Ϊ��26.09%��
���� ���⿼��ʵ�鷽������ơ�������ɼ������ⶨ���㣬��Ŀ�Ѷ��еȣ���ֿ���ѧ���ķ�������������֪ʶǨ��Ӧ��������


 ���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
���ɿ��õ�Ԫ������ĩר����100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 30g HCHO����������Ƶ�������ͭ����Һ��Ӧת����4NA������ | |
| B�� | 1L 1mol/L ������Һ�з�������С��NA | |
| C�� | ��1mol-CHO������������Ϊ15NA | |
| D�� | 1mol CaHxNbOc���л����У�H��ࣨ2a+b+2��NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ȼ�������ȼ������SO3 | |
| B�� | �й��Ŵ�����������Һ���������ͭ�������ͭ�� | |
| C�� | ���ð�˾ƥ�ֳ���ˮ���ᷴӦʱ����NaHCO3��Һ�ⶾ | |
| D�� | ʹ�ú�������Ũ�Ƚϴ�ĵ���ˮϴ�·�������ȥ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������ˮ��Cl2+H2O=2H++Cl-+ClO- | |
| B�� | Na2CO3��Һ��CO32-��ˮ�⣺CO32-+H2O=HCO3-+OH- | |
| C�� | ������Һ��KIO3��KI��Ӧ����I2��IO3-+I-+6H+=I2+3H2O | |
| D�� | NaHCO3��Һ�м�����Ba��OH��2��Һ��HCO3-+Ba2++OH-=BaCO3��+H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| t/s | 0 | t1 | t2 | t3 | t4 |
| n��so3��/mol | 0 | 0.6 | 1.2 | 1.8 | 1.8 |
| A�� | ��Ӧ��ǰt1s��ƽ������v��O2��=0.3/t1 mol•L-1•s-1 | |
| B�� | ���¶��·�Ӧƽ�ⳣ��Ϊ1.62��10-3L/mol | |
| C�� | ��ͬ�¶��£���ʼʱ�������г���4 mol SO3���ﵽƽ��ʱ��SO3��ת���ʴ���10% | |
| D�� | �¶Ȳ��䣬����������ٳ���0.2 mol SO2��0.1 mol O2��1.8 mol SO3��Ӧ�ﵽ��ƽ��ʱSO3ת�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
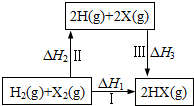
| A�� | 2H��g��+2X��g���T2HX��g����H3��0 | |
| B�� | ��H1=��H2+��H3 | |
| C�� | Cl��Br��I�ķǽ��������μ���������;�������յ�������Cl��Br��I��˳���������� | |
| D�� | ;��������HCl�ų�������������HBr�Ķ࣬˵��HCl��HBr�ȶ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
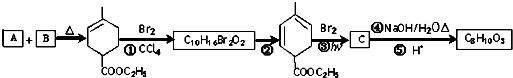
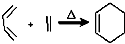 RCH2CH=CH2$��_{hv}^{Br_{2}}$RCHBrCH=CH2���밴Ҫ��ش��������⣺
RCH2CH=CH2$��_{hv}^{Br_{2}}$RCHBrCH=CH2���밴Ҫ��ش��������⣺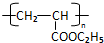 ��
��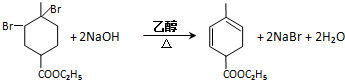 ��
�� ��2������Ӧ�ܵĻ�ѧ����ʽΪ
��2������Ӧ�ܵĻ�ѧ����ʽΪ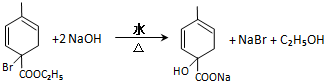 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
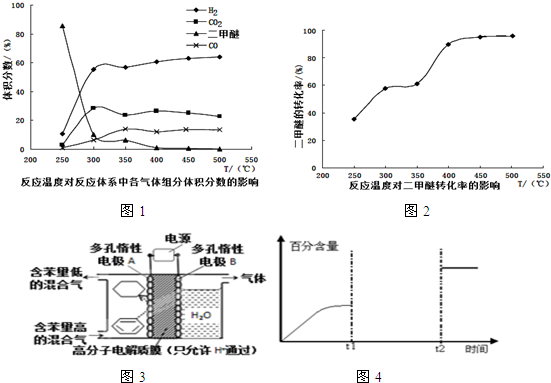
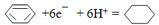 ��
���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com