
ʵ�����Կ�����O2�������20%)Ϊԭ�ϣ�����̼����ˮ�Ļ����£�����ͼAװ���Ʊ�������3O2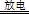 2O3����
2O3����
��1������ͨ��Aװ��֮ǰ��Ӧ�Ⱥ�ͨ������װ���е� �� ����װ����ţ���
��2��������⻯����Һ��ӦΪ��2KI+O3+H2O=2KOH+I2+O2����a������ͨ��װ��B����Һ�е�����Ϊ ��
��3��Ϊ�ⶨO2ת��ΪO3��ת���ʣ���װ��B�е���Һȫ��ת����һ�����У�����CC14������ȡ����Һ��������ȴ�����أ���I2����0.254g��
����ȡ�������ò������������� ��
����ʵ��ʱͨ�����1.12L����״������O2��ת����Ϊ ��
�۲ⶨʱ����A��Bװ�ü�����װ��D��ԭ���� ��
��4����ҵ�Ϸ���O3��O2���ɽ��������Һ�����ٷ��룬���з��뷽���������� ������ţ���
A�����ˡ�B������C����Һ��D����ȡ
��5�����������ں�CNһ���Ե�Ʒ�ˮ�Ĵ�������i����CNһת��ΪOCN������ii����OCNһ����ת��ΪCO32һ�����ֵ������塣����ii��ת��ʱ��O3��OCN�����ʵ�����֮��Ϊ3��2���ò���Ӧ�����ӷ���ʽΪ ��
��15�֣���1��D��C����1�֣�2�֣�
��2����Һ���ɫ�����������𰸸��֣�2�֣�
��3���ٷ�Һ©����1�֣�
��15%��3�֣�
�۳�ȥ��������еĵ���������������𰸸��֣�2�֣�
��4��B��2�֣�
��5��2OCN��+2OH��+3O3=2CO32һ+N2+3O2+H2O��3�֣�
���������������1��������̼��ˮ���������������ͨ��Aװ��ǰӦ�Ⱥ�ͨ��NaOH��Һ��ŨH2SO4��ϴ��ƿ����2��a������ͨ��B������������KI����Һ�������ɵ�I2��ƣ���3������ȡʹ�÷�Һ©�����ձ�������m/M��֪n(I2)=0.254g��254g/mol=0.001mol����2KI+O3+H2O=2KOH+I2+O2��֪��n(O3)=0.001mol����3O2 2O3��֪��n(O2)=0.0015mol����V/Vm��֪n(����)=0.05mol���ɿ�������ɿ�֪��n(O2)=0.01mol�����O2��ת����Ϊ0.0015��0.01��100%=15%����NaOH��Һ�ܳ�ȥ�����е����������ڷŵ�ʱ�����ĵ��������ֹNO2��KI��Һ��Ӧ����4��������������Һ�������߷е㲻ͬ�����÷���ķ������룬��B��ȷ����5����һ����Ӧ��̼Ԫ����+2����Ϊ+4�ۣ��ڶ�����Ӧ�е�Ԫ���ɡ�3����Ϊ0�ۣ�������Ԫ����0�۽�Ϊ��2�ۣ���������Ӧ�ֱ�ΪO3+CN��=OCN��+O2��2OCN��+2OH��+3O3=2CO32һ+N2+3O2+H2O��
2O3��֪��n(O2)=0.0015mol����V/Vm��֪n(����)=0.05mol���ɿ�������ɿ�֪��n(O2)=0.01mol�����O2��ת����Ϊ0.0015��0.01��100%=15%����NaOH��Һ�ܳ�ȥ�����е����������ڷŵ�ʱ�����ĵ��������ֹNO2��KI��Һ��Ӧ����4��������������Һ�������߷е㲻ͬ�����÷���ķ������룬��B��ȷ����5����һ����Ӧ��̼Ԫ����+2����Ϊ+4�ۣ��ڶ�����Ӧ�е�Ԫ���ɡ�3����Ϊ0�ۣ�������Ԫ����0�۽�Ϊ��2�ۣ���������Ӧ�ֱ�ΪO3+CN��=OCN��+O2��2OCN��+2OH��+3O3=2CO32һ+N2+3O2+H2O��
���㣺�������ʵ��Ʊ���KI����Ҫ���ʡ���ȡ������ת���ʵļ��㡢��������IJ����ʹ���������������ԭ��Ӧ�����ӷ���ʽ����д�����֪ʶ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
��ҵ���Ի�ͭ����Ҫ�ɷ���CuFeS2�����ʲ�����ˮ���ᣩΪԭ�ϣ��Ʊ���ɫ����G���仯ѧʽΪ[Cu(NH3)4]SO4��H2O���漰�������£�
��֪25��ʱ�����ֽ�������������ܶȻ���������ȫ������pH��Χ���±���
��1����ͭ���ڿ����б�������������ͭ�ĵͼ����д���䷴Ӧ�Ļ�ѧ����ʽ ��
��2���Լ�X�Ļ�ѧʽΪ ��˫��ˮ�������� ��
��3�������£�0.1 mol��L�Լ�Y��pH=11������¶��£��Լ�Y�ĵ��볣��Ϊ ����pH��ֽ�����ҺpHֵ�ķ����� ��
��4������ҺN�м����Ҵ���Ŀ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���ڼ���ʱ���뵪����������Ӧ���⻯����ˮ������Ӧ�����������ƺ��������⻯��ͨ��������������Ƽ�����ȡ��ͼ1�Ǻ�����ȡװ�á�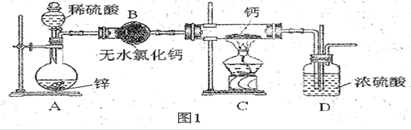
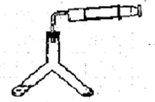
��1����Ũ��������l��4(�����)��ϡ���ᣬ���õIJ����������ձ���________��
��2��װ��D����ֱ���ܵ�������________________________��
��3��Ϊ��ȷ�Ͻ���װ��C�������Ѿ��������B��C֮���ٽ�һװ�ã���װ���м�����Լ���_______������Cװ��ǰҪ��H2�鴿�������ǣ��ռ�һ�Թ����壬���ܿڿ����ƾ��ƻ��棬�����������ۡ���������˵��H2__________________________��
��4����ͬѧ��ΪֻҪװ�ú����������淶�Ϳ����ų�����__________(ѡ�����)��
a��Ca3N2 b��CaO c��Ca(OH)2
��5����ͬѧ����ͼװ�òⶨ�Ƶõ��⻯�ƵĴ��ȡ�����ȡ46 mg��Ʒ��������ˮ��Ӧ������ʱ��ע������������������Ϊ48.06 mL(�ѻ���Ϊ��״��)������ʵ������ԭ�������_______��ѡ���ţ���
��H2ͨ�벻�㣬��Ӧ�����п���
�ڸ���H2δ��ַ�Ӧ
�۲���������Ӵ�
��6����ͬѧ������ͬѧ��ʵ����������һ����ϵʽ��42x+40y=0.046��2x+y=48.06/22400��ָ��ʽ����y�ĺ���___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�ܣ�Co������������һ����Ҫ�Ļ���ԭ�ϣ���ҵ������CoCO3+O2��CoxOy+ CO2��Ӧ��������Ӧ���ܵ������ʵ�����п���������װ������ȡ�ܵ������ﲢ�ⶨ�������ɡ�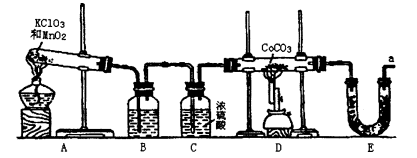
����д���пհף�
��1��д��Aװ�õĴ��Թ������Ӧ�Ļ�ѧ����ʽ ��
��2��Eװ�õ�U�ι���ʢ�ŵ������� ��
A��P2O5 B����ˮCaCl2 C����ʯ�� D����ˮCuSO4
��3��O3�������Ա�O2ǿ����֪�Ƶõ�O2�к���������Cl2��O3����Bװ������ʢ�ŵ�������
A��NaOH��Һ B������NaHCO3��Һ C������NaCI��Һ D��KI��Һ
��4��ʵ�����ʱ�����ȳ�ȥAװ���еľƾ��ƣ������� ��
��5����CoCO3��ȫת��ΪCoxOy�����Ƶ�E������4.40g��D���ڲ������ʵ�������8.30g��������CoxOy�Ļ�ѧʽΪ ��
��6����ʵ��װ�ô���һ���Ƚϴ��ȱ�ݣ�������� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
������ʵ���������ÿ�����þ��Ϊԭ����ȡ��������þ(Mg3N2)����֪ʵ���п��ܻᷢ�����з�Ӧ��
��2Mg+O2 2MgO����3Mg+N2
2MgO����3Mg+N2 Mg3N2����2Mg+CO2
Mg3N2����2Mg+CO2 2MgO+C��
2MgO+C��
��Mg+H2O MgO+H2���� ��Mg3N2 +6H2O
MgO+H2���� ��Mg3N2 +6H2O 3Mg(OH)2+2NH3��
3Mg(OH)2+2NH3��
�ɹ�ѡ���װ�ú�ҩƷ����ͼ��ʾ(þ�ۡ���ԭ���۾��Ѹ��װ�����������ķ�Ӧ����ȫ�ģ�����װ�õ�ĩ������������)��
�ش��������⣺
��1�������ʵ�鷽��ʱ����װ��A��D��E�⣬��Ӧѡ���װ���� ������ĸ���ţ���ѡ��װ��DĿ��Ϊ_____________________________ ��
��2��ͨ����Ӧ�ȵ�ȼ ���ľƾ��ƣ����ͬʱ��ȼA��Fװ�õľƾ��ƣ�����ʹʵ���� ���ƫ�ߡ���ƫ�͡���ԭ��
��3�������һ��ʵ�飬��֤������Mg3N2��д���������衢����ͽ��ۣ�
_____________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
���������ǻ���ɫ����ǿ�Ҵ̼��Ե����塣�����۵�-116�棬�е�3.8�森�������Ȳ��ȶ����Ӵ�һ���л����ױ�ը����������ˮ(1��100)ͬʱ��Ӧ���ɴ�������Һ����ȡ�����������ȣ����ø������������������Ӧ(������HgO��HgCl2)��װ����ͼ��������̨�ͼг���������ȥ��
�������ʵ��й������������£�
| ��ѧʽ | �۵�(��λ����) | �е�(��λ����) |
| N2 | -209.86 | -195.8 |
| O2 | -218.4 | -183 |
| CO2 | -57 | / |
| NH3 | -77.3 | -33.35 |
| Cl2 | -101 | -34.6 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
(14��)ij��ѧ�С���������ͼ��ʾ(���ּг�װ������ȥ)ʵ��װ�ã���̽����ʪ��Cl2��Na2CO3��Ӧ�õ��Ĺ������ʡ�
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ__________________________________________��
��2��װ��B���Լ�YӦΪ__________________________________��
��3����ʵ��װ�������Դ��ڲ���֮�����Ľ��Ĵ�ʩΪ_____________________________��
��4����֪��װ��C��ͨ��һ�������������D��ֻ��һ�ֳ�����Ϊ�ƺ�ɫ������(����������)��C�к���Ԫ�ص���ֻ��һ�֣��Һ���NaHCO3���ֶ�C�еijɷֽ��в����̽����
������������衣
����һ���������ֳɷ֣�ΪNaHCO3��____________��
��������������ֳɷ֣�ΪNaHCO3��_____________��______________��
����Ʒ�����ʵ�顣���ڱ�����д��ʵ�鲽���Լ�Ԥ������ͽ��ۡ�
��ѡ�Լ�������������ˮ��ϡ���ᡢBaCl2��Һ������ʯ��ˮ��AgNO3��Һ���Թܡ�С�ձ���
���ۣ��ɲ���3�Ľ��۽�ϲ���2�е�a�������һ�������ɲ���3�Ľ��۽�ϲ���2�е�b��������������
��5����֪C����0.1 mol Cl2�μӷ�Ӧ��������һ����������֪C�з�Ӧ���ɵĺ���������Ϊ_________(д��ѧʽ)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
����װ��Ϊ��ѧ��ѧʵ�鳣�õ������Ʊ�װ��


A B C
��1������NaHCO3��ϡ������ȡ������̼��Ӧѡ��װ�� ��
��2������NH4Cl�ͼ�ʯ����ȡNH3��Ӧѡ��װ�� ��
��3�����ø�����غ�Ũ���������ȡCl2��Ӧѡ��װ�� ��
��4����ij�о���С��������ϵ�֪��Ư����������Һ��Ӧ���Ƶ���������ѧ����ʽΪ��Ca(ClO)2+CaCl2+2H2SO4="2" CaSO4+2 Cl2+2H2OӦѡ��װ�� ��
��Ϊ֤���������������Խ������ֱ�ͨ�뵽����������Һ���Ȼ�������Һ�У����ʵ��֤�����������Ѿ������� ��
д���������Ȼ�������Һ��Ӧ�����ӷ���ʽ�� ��
����д������β�����������ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com