
 ��
������ ��1���õ��⻯������N��H��=$\frac{32��12.5%}{1}$=4���ʷ�����N��N��=$\frac{32-4}{14}$=2���õ��⻯����ΪN2H4����������ԭ��֮���γ�1�Թ��õ��Ӷԣ���ԭ���뵪ԭ��֮���γ�1�Թ��õ��Ӷԣ��ݴ���д�����ʽ�ͽṹʽ��
��2��N2H4��Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ���������ʣ������ɵ�����ˮ��
��3��N2H4������ÿ��Nԭ�Ӷ�����1�Թ¶Ե��ӣ���1molN2H4��2molHCl��Ӧ����N2H6Cl2��
��� �⣺��1���õ��⻯������N��H��=$\frac{32��12.5%}{1}$=4���ʷ�����N��N��=$\frac{32-4}{14}$=2���õ��⻯����ΪN2H4����������ԭ��֮���γ�1�Թ��õ��Ӷԣ���ԭ���뵪ԭ��֮���γ�1�Թ��õ��Ӷԣ�����ʽΪ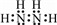 ���ṹʽΪ��
���ṹʽΪ�� ��
��
�ʴ�Ϊ��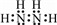 ��
�� ��
��
��2��N2H4��Һ̬˫��ˮǡ����ȫ��Ӧ�������������ֲ���Ⱦ���������ʣ������ɵ�����ˮ���÷�Ӧ��ѧ����ʽΪ��N2H4+2H2O2=N2+4H2O��
�ʴ�Ϊ��N2H4+2H2O2=N2+4H2O��
��3��N2H4������ÿ��Nԭ�Ӷ�����1�Թ¶Ե��ӣ���1molN2H4��2molHCl��Ӧ����N2H6Cl2����Ӧ����ʽΪ��N2H4+2HCl=N2H6Cl2��
�ʴ�Ϊ��N2H4+2HCl=N2H6Cl2��
���� ���⿼��������й��ƶϡ����û�ѧ����ȣ��Ѷ��еȣ���ȷ���ʵĽṹ�ص��ǽ���ؼ���ע��������Ϣ���գ�


 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH3CH2Br$��_{ˮ}^{NaOH}$ CH3CH2OH$��_{170��}^{Ũ����}$ CH2=CH2$\stackrel{Br_{2}}{��}$ CH2BrCH2Br | |
| B�� | CH3CH2Br $\stackrel{HBr}{��}$ CH2BrCH2Br | |
| C�� | CH3CH2Br $��_{ˮ}^{NaOH}$ CH2=CH2 CH2BrCH3$\stackrel{HBr_{2}}{��}$CH2BrCH2Br | |
| D�� | CH3CH2Br $��_{��}^{NaOH}$ CH2=CH2$\stackrel{Br_{2}}{��}$ CH2BrCH2Br |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ��NaCl�����У�ÿ��Na+��Χ������������Na+��6�� | |
| B�� | �����Ӳ�ȡA2�ܶѻ�������������������϶�� | |
| C�� | ÿ��������2��Na+��2��Cl- | |
| D�� | �Ȼ��ƵĻ�ѧʽΪNaCl |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��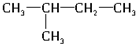 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ԭ�ӵĽṹʾ��ͼ��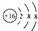 | |
| B�� | HCl�ĵ���ʽ�� | |
| C�� | ����Ľṹʽ��C2H4O2 | |
| D�� | �����Ƶĵ��뷽��ʽ��Na2SO4=2Na++SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
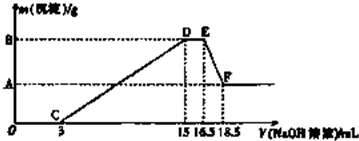
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CH4 | B�� | C2H6 | C�� | C6H6 | D�� | C6H6O2 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com