


 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
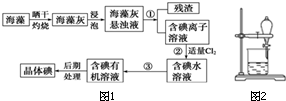 ����ֲ���纣���������к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ������Ӻ�������ȡ���������ͼ1��
����ֲ���纣���������к��д����ĵ�Ԫ�أ���Ԫ���Ե����ӵ���ʽ���ڣ�ʵ������Ӻ�������ȡ���������ͼ1���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ԭ�Ӻ������ӵĻ�ѧ������ͬ |
| B��ʪ����������Ư���� |
| C�������ж���������Ҳ�ж� |
| D�������������Ӷ��Ի���ɫ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��ʯī�IJ�״�ṹ���ɹ��ۼ��γɵ���С��̼ԭ�ӻ���������̼ԭ�� |
| B���Ȼ��ƾ�����ÿ��Na+��Cl-��Χ���ڵ���6��Cl-��Na+ |
| C����CsCl������ÿ��Cs+��Χ���ڵ���8��Cl-������ÿ��Cs+�Ⱦ�����ڵ�Ҳ��8��Cs+ |
| D�������������ܶѻ��Ľ��������У�ÿ������ԭ����Χ���ڵ���4������ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��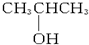 |
B��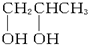 |
| C��CH3CH2CH2OH |
| D��CH3CH2OH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A�����ʯΪ�ռ���״�ṹ���ɹ��ۼ��γɵ�̼ԭ�ӻ��ϣ���С�Ļ�����6��̼ԭ�� |
| B���Ȼ��ƾ����У�ÿ��Na+��Χ������ȵ�Na+����6�� |
| C���Ȼ�菉����У�ÿ��Cs+��Χ����8��Cl- |
| D���ɱ������У�ÿ��CO2������Χ����12��CO2���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ���ᷴӦ�û����������� |
| B�������Ե�ǿ�� |
| C���۵� |
| D�����Ӳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��������ˮ |
| B�����нϸߵ��۵� |
| C��ˮ��Һ�ܵ��� |
| D�����岻���磬������״̬�ܵ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
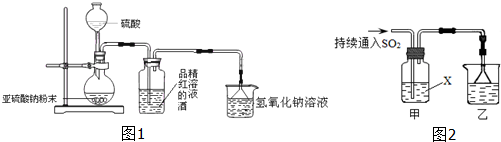
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com