
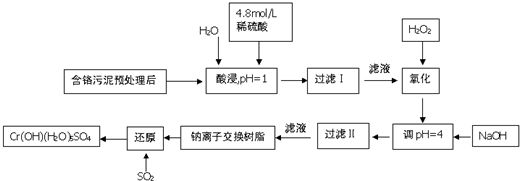
| ŃōĄė×Ó | Fe3+ | Fe2+ | Mg2+ | Al3+ | Ca2+ | Cr3+ |
| æŖŹ¼³ĮµķŹ±µÄpH | 1.9 | 7.0 | 9.6 | 4.2 | 9.7 | - |
| ³ĮµķĶźČ«Ź±µÄpH | 3.2 | 9.0 | 11.1 | 8.0 | 11.7 | 9.0£Ø£¾9.0Čܽā£© |



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢ÓĆĶ×öµē¼«µēCuSO4½āČÜŅŗ£ŗ2Cu2++2H2O
| ||||
| B”¢ŌŚNH4Fe£ØSO4£©2ČÜŅŗÖŠ£¬µĪ¼ÓÉŁĮæBa£ØOH£©2ČÜŅŗ£ŗ2NH4++SO42-+Ba2++2OH-ØT2NH3?H2O+BaSO4”ż | ||||
| C”¢ŌŚNa2CO3ČÜŅŗÖŠÖšµĪµĪČėĻ”ŃĪĖįČÜŅŗ£¬·“Ó¦æŖŹ¼½×¶Ī£ŗCO32-+H+ØTHCO3- | ||||
| D”¢ŌŚNa2S2O3ČÜŅŗÖŠ¼ÓČėĻ”ĮņĖį£ŗ2S2O32-+4H+ØTSO42-+3S”ż+2H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢ĻņijČÜŅŗÖŠ¼ÓČėĻ”ŃĪĖį£¬²śÉśÄÜŹ¹ŹÆ»ŅĖ®±ä»ė×ĒµÄĘųĢ壬øĆČÜŅŗŅ»¶Øŗ¬ÓŠCO32- |
| B”¢ĻņijČÜŅŗÖŠµĪ¼ÓĀČĖ®ŗ󣬵Ī¼ÓKSCNČÜŅŗ£¬ČÜŅŗ³ŹŗģÉ«£¬øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠFe2+ |
| C”¢ĻņijČÜŅŗÖŠ¼ÓČėĀČ»Æ±µČÜŅŗ£¬²śÉś²»ČÜÓŚĻ”ŃĪĖį°×É«³Įµķ£¬øĆČÜŅŗŅ»¶Øŗ¬ÓŠSO42- |
| D”¢ĶłÄ³ČÜŅŗÖŠ¼ÓČėNaOH£¬Ī¢ČČ£¬²śÉśÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬øĆČÜŅŗÖŠŅ»¶Øŗ¬ÓŠNH4+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
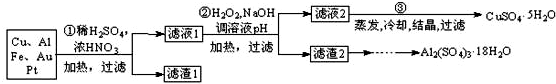
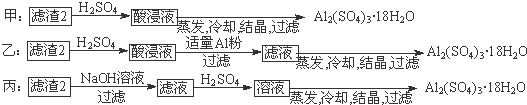
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
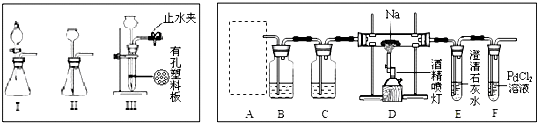
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
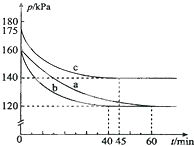 »ÆŗĻĪļAX3ŗĶµ„ÖŹX2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æÉÉś³É»ÆŗĻĪļAX5£®»Ų“šĻĀĮŠĪŹĢā£ŗ
»ÆŗĻĪļAX3ŗĶµ„ÖŹX2ŌŚŅ»¶ØĢõ¼žĻĀ·“Ó¦æÉÉś³É»ÆŗĻĪļAX5£®»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com