
���� ��1��Cu2O��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ3Cu2O+14HNO3�T6Cu��NO3��2+2NO��+7H2O��
��2����Nԭ���غ�ĽǶȼ�������ͭ�����ʵ�����
��3����������ԭ��Ӧ��ʧ�����غ�ĽǶȼ��㣻
��4�����������͵����غ��з���ʽ���㣮
��� �⣺��1��Cu2O��ϡ���ᷴӦ�Ļ�ѧ����ʽΪ3Cu2O+14HNO3�T6Cu��NO3��2+2NO��+7H2O���ʴ�Ϊ��3Cu2O+14HNO3�T6Cu��NO3��2+2NO��+7H2O��
��2������Nԭ���غ��֪��n��Cu��NO3��2��=$\frac{0.1L��0.6mol/L-\frac{0.224L}{22.4L/mol}}{2}$=0.025mol���ʴ�Ϊ��0.025��
��3����CuԪ���غ�ɵã�2��n��Cu20��+n��CuO��+0.01mol=0.025mol��
��������ԭ��Ӧ��ʧ�����غ��֪��0.01��2+2��n��Cu20��=$\frac{0.224L}{22.4L/mol}$��
��֮�ã�n��Cu20��=0.005mol��n��CuO��=0.005mol��
�ʴ�Ϊ��0.005mol��
��4����CuԪ���غ�ɵã�2��n��Cu20��+n��CuO��+X=0.025mol��
��������ԭ��Ӧ��ʧ�����غ��֪��X��2+2��n��Cu20��=$\frac{0.224L}{22.4L/mol}$��
��֮�ã�n��CuO��=X-0.005mol��
n��Cu20��=0.015mol-X��
��0.005mol��X��0.015mol��
�ʴ�Ϊ��0.005mol��X��0.015mol��
���� ���⿼������ļ��㣬��Ŀ�Ѷ��еȣ�ע��������غ��������ԭ��Ӧ��ʧ�����غ�ĽǶȷ�����


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��⾫��ͭ�Ĺ����У�ÿת��NA������ʱ�������ܽ�ͭ������Ϊ32g | |
| B�� | һ��������6.4gS02������������Ӧ����S03��ת�Ƶ�����С��0.2NA | |
| C�� | lmol�ǻ���һOH����17gNH3������������ΪNA | |
| D�� | ����ͭ���ܽ���1 L0.5mol/Lϡ�����У�������2.24LNOʱ����Һ�е�ԭ����0.4NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��Ũ����ȡ������Ϊ50mL��A��B����NaOH��Һ�У��ֱ�ͨ��һ������ CO2����ϡ�͵�100mL��
��Ũ����ȡ������Ϊ50mL��A��B����NaOH��Һ�У��ֱ�ͨ��һ������ CO2����ϡ�͵�100mL���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢ۢ� | C�� | �ڢܢ� | D�� | �٢ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 �����й���ͼ��ʾװ�õ������д�����ǣ�������
�����й���ͼ��ʾװ�õ������д�����ǣ�������| A�� | �٢� | B�� | �ۢ� | C�� | �٢� | D�� | �ڢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ڿ����г��ֺ�ɫ�����⣬�为����ӦʽΪ��Fe-3e-�TFe3+ | |
| B�� | ������þ��ɫ���������Ȼ����Һ��Mg��OH��2+NH4+�TMg2++NH3•H2O | |
| C�� | ����������������������Һ��Ӧ�����Һ�еμ�̼��������Һ��HCO3-+AlO2-+H2O�TAl��OH��3��+CO32- | |
| D�� | Ư����Һ�м��Ȼ�������Һ��������������Fe2++2ClO-+2H2O�TFe��OH��2��+2HClO |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �μ�������NaOH��Һ����ϴ�����գ����չ���Ϊ72g | |
| B�� | �������ϡ���ᣬ�����������������ܱ�ɺ���ɫ | |
| C�� | ���˹�����ϡ�����KSCN��Һ����Һ��Ѫ��ɫ | |
| D�� | ����Һ�������������ǣ�Fe2+��Na+��SO42-��NO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
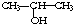 ��NaOH�Ĵ���Һ�����Ʊ�CH3-CH�TCH2
��NaOH�Ĵ���Һ�����Ʊ�CH3-CH�TCH2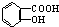 ������NaHCO3��Һ��Ӧ�Ʊ�
������NaHCO3��Һ��Ӧ�Ʊ�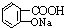
| A�� | ֻ�Т٢ۢ� | B�� | ֻ�Т٢� | C�� | ֻ�Т� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ����� | ʵ������ | ���ӷ���ʽ |
| A | ��NH4��2SO4��Һ�м�������Ba��OH��2��Һ������ | ����ʹʪ��ĺ�ɫʯ����ֽ���������� | NH4++OH- $\frac{\underline{\;\;��\;\;}}{\;}$NH3��+H2O |
| B | ���ȵ��з�̪��Na2CO3��Һ | ��Һ��ɫ���� | CO32-+2H2O�TH2CO3+2OH- |
| C | �ö��Ե缫����Ȼ�þ��Һ | �а�ɫ��������ɫ��ζ�������� | 2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$2OH-+H2��+Cl2�� |
| D | ��Na2S2O3��Һ�м���ϡ���� | ��Һ���ֻ��ǣ�������ɫ�̼�����ζ���� | 2H++S2O32-�TS��+SO2��+H2O |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com