
(12��)ij̽��С�齫һ����������·������õ���70��Cu��25��Al��4��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�
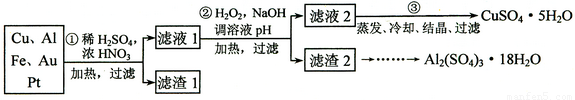
��ش��������⣺
�� �ڢٲ�Cu���ᷴӦ�����ӷ���ʽ______________ ��
�� �ڢڲ���H2O2��������_______________������ҺpH��Ŀ����ʹ____________���ɳ�����
�� �õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ�����______________��
�� ������2��ȡAl2(SO4)3��18H2O ��̽��С����������ַ�����
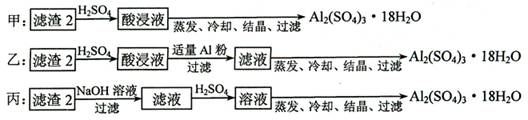
�������ַ����У�_______���������У�ԭ����_____________________________��
��ԭ�������ʽǶȿ��ǣ�___________������������
�� ̽��С���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol /L EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һ6 mL���ζ���Ӧ���£�Cu2+ + H2Y2�� �� CuY2�� + 2H+
д������CuSO4��5H2O���������ı���ʽ�أ� _____________________________ ��
���в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
��12�֣��𰸣��� Cu + 4H+ + 2NO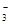
 Cu2+
+ 2NO2��+ 2H2O ��3Cu + 8H+ + 2NO
Cu2+
+ 2NO2��+ 2H2O ��3Cu + 8H+ + 2NO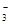
 3Cu2+
+ 2NO��+ 4H2O ��2�֣�
3Cu2+
+ 2NO��+ 4H2O ��2�֣�
�� ��Fe2+����ΪFe3+ Fe3+��Al3+
�� ������ˮ
�� �� ���ò�Ʒ�к��н϶�Fe2(SO4)3���� ��
�� �� 100% ��2�֣� c ��2�֣�
������������������ﴦ��ʱ����1��ͭ��Ũ���ᡢϡ����Ļ���
��ʼ������Cu+2NO3-+4H+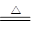 Cu2++2NO2��+2H2O��
Cu2++2NO2��+2H2O��
�������3Cu+2NO3-+8H+ 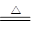 3Cu2++2NO��+4H2O�������������ֻ��Au��Pt���������ᣬ������������Ҫ�ɷ֣�
3Cu2++2NO��+4H2O�������������ֻ��Au��Pt���������ᣬ������������Ҫ�ɷ֣�
��2���ڢڲ�������������Ŀ���ǽ�Fe2+����ΪFe3+����������������������ʱ���ɵ��������Ӻ�ˮ�����������ʣ��Ի�������Ⱦ������pHֵ��ʹ��Һ�е�Fe3+��Al3+ת��Ϊ������
��3���õڢ۲������õ���ˮ����ͭ��ȡ��ˮ����ͭ�ķ����Ǽ�����ˮ��
��4������2����Ҫ�ɷ������������������������Դ�Ϊ����������̽��С����Ƶ����ַ��������з��������Ƶò�Ʒ��һ�����н϶��Fe2(SO4)3���ʣ������Һͱ��У���ԭ�����õĽǶȿ��ǣ��ҷ����������
��5������̽��С��ķ�������֪������ˮ����ͭ���������ı���ʽΪ��
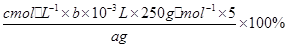 ��
��
�ζ������У�δ������ƿ���Խ����Ӱ�죬�ų�a���ζ��յ�ʱ�ζ��ܼ����в������ݣ��������EDTA�Ķ���ƫС���ⶨ���ƫ�ͣ��ų�b��δ�����������ӣ����EDTA����ƫ�࣬�ⶨ���ƫ�ߣ�ѡc��


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12�֡�ij�о���ѧϰС����̽��SO2�ܷ���BaCl2��Һ��Ӧ����BaSO3�������������ϵ�֪������BaSO3��KSPΪ��������������
��
(1)��0.1 mol• L��1��BaCl2��Һ���뱥���������У�_______(��ܡ����ܡ�������BaSO3������ԭ����______________(��д����Ҫ���ƶϹ��̣���
(2)Ũ����ķе�Ϊ338��C���ƾ��ƻ�����¶�Ϊ400〜5000C����ͬѧ��װ��I����ʵ�飬����BaCl2��Һ�г��ְ�ɫ�������Ұ�ɫ�������������ᡣ
��д�������Թ��з�����Ӧ�Ļ�ѧ����ʽ��_____________________
�ڰ�ɫ�����Ļ�ѧʽ��_______���������ӷ���ʽ��ʾ���ɸð�ɫ�����Ŀ���ԭ��___________________________________
(3)��ͬѧ��Ϊ��ͬѧ��װ�ò����ƣ�����˸Ľ�װ��II����ʵ�飨�г�װ�ú�A�м���װ�����ԣ��������Ѽ��飩��
�ٴ��ɼУ�ͨ��N2����ʱ���رյ��ɼ�
�ڵμ�һ����Ũ���ᣬ����A��һ��ʱ���C��δ���������ɡ�
�����ٵ�Ŀ����_______��ϴ��ƿB�е��Լ���______________��
(4)��ͬѧȡ��ʵ����C����Һ�������μ�һ����ɫ��Һ��Ҳ��������������İ�ɫ���������μӵ��Լ�������______________��
A. NaOH ��Һ B. Na[Al(OH)4]��Һ C. H2O2 ��Һ D.���� KMnO4 ��Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����12�֣�ij�о���ѧϰС��Ϊ�о�Cu��ŨH2SO4�ķ�Ӧ���������ʵ��̽��������װ���еĹ̶������;ƾ��ƾ�δ������
ʵ��ѡ��ϸͭ˿��98.3% H2SO4��Ʒ����Һ������ʯ��ˮ��CCl4��NaOH��Һ��ҩƷ��ͭ˿����������״��һ��û��ŨH2SO4�У���һ��¶����Һ���Ϸ���
|
�����������ϻش���������
��1��A�Թ��Ϸ��ij����ܵ�������_________________��D��E��֧�Թ���CCl4��������_____________��
��2�����ȹ����У��۲쵽A�Թ��г��ִ�����ɫ��������������������Թ��ϲ��ڱ���������ɫ�������ʣ��ڳ�������Ũ���ᣨ���ڣ�ʱ������ɫ������������������ʧ��д������ɫ������ʧ�Ļ�ѧ��Ӧ����ʽ��____��
��3����A�Թ��е�ŨH2SO4��ͭ˿���м��ȣ��ܿ췢��C�Թ���Ʒ����Һ��ɫ����ʼ��δ��D�Թ��г���ʯ��ˮ���ֻ��ǻ��������IJ����ǣ�___________�����ʵ����֤��IJ���________________��
��4�����������о��������ѧ֪ʶ������ΪҺ���·�ͭ˿����ĺ�ɫ���ʳɷ���_____����д��ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�������ʡ����ʵ����ѧ������ѧ�ڿ�ѧ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(12��)ij̽��С�齫һ����������·������õ���70��Cu��25��Al��4��Fe������Au��Pt�Ƚ����Ļ�������Ƴ������Ʊ�����ͭ�������������·�ߣ�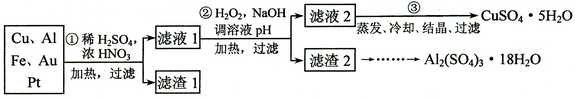
��ش��������⣺
�ŵڢٲ�Cu���ᷴӦ�����ӷ���ʽ______________ ��
�Ƶڢڲ���H2O2��������_______________������ҺpH��Ŀ����ʹ____________���ɳ�����
���õڢ۲�����CuSO4��5H2O�Ʊ���ˮCuSO4�ķ�����______________��
��������2��ȡAl2(SO4)3��18H2O ��̽��С����������ַ�����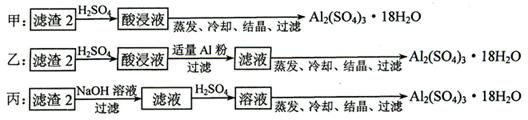
�������ַ����У�_______���������У�ԭ����_____________________________��
��ԭ�������ʽǶȿ��ǣ�___________������������
��̽��С���õζ����ⶨCuSO4��5H2O������ȡa g�������100 mL��Һ��ÿ��ȡ20.00 mL�������������Ӻ���c mol /L EDTA��H2Y2��������Һ�ζ����յ㣬ƽ������EDTA��Һ6 mL���ζ���Ӧ���£�Cu2+ + H2Y2���� CuY2�� + 2H+
д������CuSO4��5H2O���������ı���ʽ�أ� _____________________________ ��
���в����ᵼ��CuSO4��5H2O�����IJⶨ���ƫ�ߵ���_____________��
a��δ������ƿ
b���ζ��յ�ʱ�ζ��ܼ����в�������
c��δ��������EDTA��Ӧ�ĸ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��08ï���ж�ģ��������12�֣�ijѧϰС��Ϊ̽��S��Cl2�ܷ�Ӧ���������ϻ����Ϣ�������Cl2��110�桫140����S��Ӧ���ɵ�S2Cl2��Ʒ���ɴ������������װ�ã��г�װ��δ����������ش�������⡣

(1)ʵ������У�ʹ�÷�Һ©���μ�Ũ����IJ����� ��2�֣���2���÷�Ӧ����ʽ��ʾHװ�õ����� ��2�֣���3��д��B�з�Ӧ�����ӷ���ʽ ��2�֣���4��C��D�е��Լ��ֱ��� �� ��2�֣���5��������Ʒ�к���SCl2��Ϊ����SCl2�����ɣ��ؼ��IJ����� ��2�֣���6��F���ܿ��ܻᷢ������������C��D֮�����һ��������װ�����ⷢ��Σ�ա���Ҫ��װ��ͼ������Լ����ƣ��г�װ�ò��軭���� ��2�֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com