
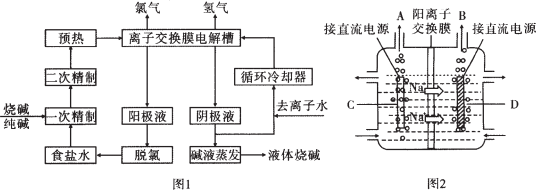
���� ��1�����ݴ��������ˮ�л����������������Ӷ�װ�õ�Ӱ��Ƕ����ش𣻳�ȥ������ʹ��̼�����Լ�����ȥþ����ʹ�����������Լ���
��2�����ݹ�������ͼ��ϵ��յ�ԭ���Լ�����֪ʶ���жϣ�
��3���������������ԣ��ܽ�������������Ϊ�����ƣ�
��4������ͼװ�÷���������������D����˵��DΪ������CΪ������D�缫�����������������������������Ƴ�ֽӴ���������Ǵ������ƺ�������
��5����������NaOH�������������ۺĵ��������ʵ�ʺĵ������㣮
��� �⣺��1����ȥ������ʹ��̼�����Լ���Ca2++CO32-=CaCO3����ȥþ����ʹ�����������Լ���Mg2++2OH-=Mg��OH��2������ʳ��ˮ���������ξ��ƣ����������ˮ�л�����������������Mg2+��Ca2+�����������»����ɳ�������װ���еĽ���Ĥ����Ӱ�죬���Ծ���ʳ��ˮ��Ŀ���Ƿ�ֹ��Ĥ��������߲�Ʒ�Ĵ��ȣ�
�ʴ�Ϊ����ֹ��Ĥ��������߲�Ʒ�Ĵ��ȣ�Ca2++CO32-=CaCO3����Mg2++2OH-=Mg��OH��2����
��2�����ݹ�������ͼ�����õ���ԭ���Լ��������֪���������۳����ĵ���ˮ�к����Ȼ��ƿ���ѭ��ʹ�ã����������������ƿ���Ϊ�ڽ������ǰ��Ҫ�������ξ��Ƶ�ԭ�ϣ�Ҳ�ǿ���ѭ��ʹ�õ����ʣ�
�ʴ�Ϊ���Ȼ��ơ��������ƣ�
��3���������������ԣ��ܽ�������������Ϊ�����ƣ���Na2SO3+Cl2+H2O=Na2SO4+2HCl��
�ʴ�Ϊ��Na2SO3+Cl2+H2O=Na2SO4+2HCl��
��4������ͼװ�÷���������������D����˵��DΪ������CΪ������D�缫�����ӵõ����ӷ�����ԭ��Ӧ����������������Ĥ������ʳ��ˮʱ��2NaCl+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+NaOH�����������������Ƴ�ֽӴ���Cl2+2NaOH=NaCl+NaClO+H2O��������Ǵ������ƺ��������������ܷ�ӦΪ��NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
�ʴ�Ϊ��H2��������NaCl+H2O$\frac{\underline{\;���\;}}{\;}$NaClO+H2����
��5��m��NaOH��=b��106g��32%=3.2b��104g��
����������Ҫ������Ϊ I=$\frac{3.2b��1{0}^{4}}{a}$A��
��ʵ���Ϻĵ���Ϊ300��8��C=2400CA��
�õ��۵ĵ��Ч��Ϊ$\frac{3.2b��1{0}^{4}}{\frac{a}{2400C}}$��100%=$\frac{400b}{3ac}$��100%��
�ʴ�Ϊ��$\frac{400b}{3ac}$��100%��
���� �����ۺϿ��麣ˮ��Դ�����ã�Ϊ��Ƶ���㣬������ѧ���ķ��������ͼ��������Ŀ��飬ע����յ���ԭ������Ϸ�Ӧ����ط���ʽ���㣬�Ѷ��еȣ�


 ������ÿ�ʱ��ҵϵ�д�
������ÿ�ʱ��ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ���� | ���� | ������Ӧѡ�õ��Լ���������� |
| A | CCl4 | Br2 | ��NaOH��Һϴ�ӡ���Һ |
| B | FeCl3 | CaCO3 | �ܽ⡢���ˡ������ᾧ |
| C | Al2��SO4��3��Һ | MgSO4 | ��������ռ����ˣ����������ữ��Һ |
| D | CO2 | SO2 | ͨ��ʢ��Ʒ����Һ��ϴ��ƿ����ͨ��ʢ��Ũ�����ϴ��ƿ |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
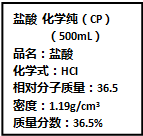 ��Ҫ���������С�⣮
��Ҫ���������С�⣮�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
 ����һ�������·�Ӧ�������������У�������
����һ�������·�Ӧ�������������У�������| A�� | 2�� | B�� | 3�� | C�� | 4�� | D�� | 5�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
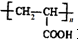 ���ĺϳ�·�ߣ�
���ĺϳ�·�ߣ�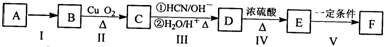
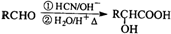 ��R������
��R������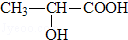

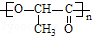 +nH2O��
+nH2O��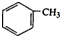 �ϳ�
�ϳ�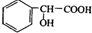 �ĺϳ�·��
�ĺϳ�·��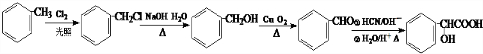 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
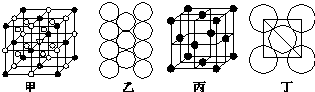
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �����ơ�ƴ�����ĭ | |
| B�� | ��N2+3H2?2NH3�ķ�Ӧ��ʹ������ý�ɼӿ�ϳɰ���Ӧ������ | |
| C�� | ��ҵ��ȡ������Na��l��+KCl��l��?NaCl��l��+K��g��ѡȡ���˵��¶ȣ�ʹK�������ӷ�Ӧ������з������ | |
| D�� | ��ҵ����������Ĺ�����ʹ�ù����Ŀ�������߶�������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ij�������ķ���ʽΪC10H14��������ʹ��ˮ��ɫ������ʹ����KMnO4��Һ��ɫ���ҷ��ӽṹ��ֻ��һ���������������������3�� | |
| B�� | 2��3-���ǻ���ȩ�����ȩ�� | |
| C�� | ���顢��ȩ�����ض�������ͬ���칹�� | |
| D�� | ij�л��ﺬ��C��H��O��N����Ԫ�أ������ģ��Ϊ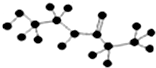 �����л���Ľṹ��ʽΪ �����л���Ľṹ��ʽΪ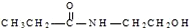 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʯ��ʯ�ڸ����µķֽⷴӦ | B�� | ���ȷ����� | ||
| C�� | ʵ���������� | D�� | ľ̿��������ȼ�� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com