
�±���Ԫ�����ڱ���һ���֡�
| �� | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| һ | �� | ||||||
| �� | �� | �� | �� | �� | �� | ||
| �� | �� | �� | �� | �� | |||
| �� | �� | �� | �� |
��1��Ԫ�� �ĵ��������³�Һ̬��Ԫ�� ���⻯�����ȶ���Ԫ�� ������������ˮ�����������ǿ��Ԫ�� �ĸ��������ˮ����ļ�����ǿ����д��Ԫ�ط��š�����
��2������Ԫ�آ�����γ��ȶ�������Ľṹʽ�� ��
��3������Ԫ�آ٢ۢ���ɵġ�A4B2C2�������ʣ������ں����������� ��
A�����Ӽ����ۼ� B����Ϊ���ۼ�
C����Ϊ���Թ��ۼ� D�����Թ��ۼ��ͷǼ��Թ��ۼ�
��4������Ԫ�� ���⻯��ķ��Ӽ�����������д��������š�����
 �п������п��Ծ����ϵ�д�
�п������п��Ծ����ϵ�д� ��������״Ԫ��ϵ�д�
��������״Ԫ��ϵ�д� �ƸԿ�����ҵ��ϵ�д�
�ƸԿ�����ҵ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������������ֿ�ʼ��Ű�ҹ��ֵ���������SO2����ɿ�����Ⱦ����Ҫԭ�������Ƽ�ѭ�����ɳ�ȥSO2��
(1)�Ƽ�ѭ�����У�����ҺΪNa2SO3��Һ�������շ�Ӧ�����ӷ���ʽ��________________��
(2)��֪H2SO3�ĵ��볣��ΪK1��1.54��10��2��K2��1.02��10��7��H2CO3�ĵ��볣��ΪK1��4.30��10��7��K2��5.60��10��11�������������Թ������ ________________��
A��CO ��HSO
��HSO B��HCO
B��HCO ��HSO
��HSO
C��SO ��HCO
��HCO D��H2SO3��HCO
D��H2SO3��HCO
(3)����Һ����SO2�Ĺ����У�pH��n(SO ):n(HSO
):n(HSO )�仯��ϵ���±���
)�仯��ϵ���±���
| n(SO | 91:9 | 1:1 | 9:91 |
| pH | 8.2 | 7.2 | 6.2 |
���ϱ��ж�NaHSO3��Һ��________________�ԣ���ԭ���ĽǶȽ���ԭ��________________��
����NaHSO3��Һ����Ũ�ȹ�ϵ����ȷ����________(ѡ����ĸ)��
A��c(Na��)��2c(SO )��c(HSO
)��c(HSO )
)
B��c(Na��)>c(HSO )>c(H��)>c(SO
)>c(H��)>c(SO )>c(OH��)
)>c(OH��)
C��c(H2SO3)��c(H��)��c(SO )��c(OH��)
)��c(OH��)
D��c(Na��)��c(H��)��2c(SO )��c(HSO
)��c(HSO )��c(OH��)
)��c(OH��)
(4)������Һ��pH����ԼΪ6ʱ����������������������ʾ��ͼ���£�
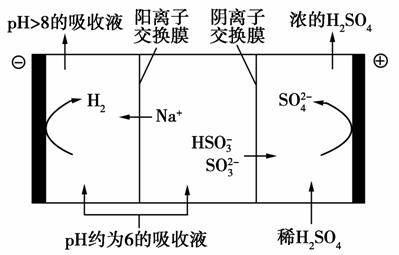
������Һ���������е��ܷ�Ӧ����ʽ��________________��
�ڵ��缫����1 mol����ת��ʱ��������Ϊ________g��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijһ��ɫ����Һ��ֻ���ܴ���H+��Ba2+��Mg2+��Cu2+��OH-��HCO3-��
CO32-��NO3-��SO42- �е�һ�ֻ��֡���֪����Һ����Al��Ӧ�ų�H2
��1������Ӧ����Al3+����ԭ��Һ��һ������ ���ܴ��ڵ�������
��2������Ӧ����AlO2- ����ԭ��Һ��һ������ ���ܴ��ڵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���л�ѧ��Ӧ�У��������Ӽ������Լ����Ǽ��Լ��Ķ��ѣ��������Ӽ������Լ����Ǽ��Լ��γɵ���
A. 2Na2O2 + 2H2O == 4NaOH + O2�� B. Mg3N2 + 6H2O == 3Mg(OH)2 + 2NH3��
C. Cl2 + H2O == HCl + HClO D. NH4Cl + NaOH== NaCl+ NH3��+ H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ͭ��һ����Ũ���ᷴӦ���õ�����ͭ��Һ��NO2��N2O4��NO�Ļ�����壬��Щ������1.68 L O2(��״��)��Ϻ�ͨ��ˮ�У�����������ȫ��ˮ�����������ᡣ������������ͭ��Һ�м���5 mol��L-1 NaOH��Һ��Cu2��ǡ����ȫ������������NaOH��Һ�����
A��60 mL B��45 mL C��30 mL D��15 mL
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й���SiO2��CO2��˵������ȷ���� (����)
A��CO2��SiO2�ֱ���̼����������
B��CO2��SiO2��ˮ��Ӧ�ֱ�������Ӧ����
C��CO2�����������ԣ�SiO2������������
D��CO2��SiO2��������Ӧ�ķ�����ɵ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
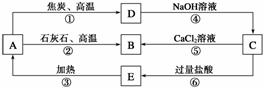
A��B��C��D��E���������о�����ͬһ�ַǽ���Ԫ�أ������ܷ�����ͼ��ʾ��ת����ϵ����Ԫ��(��R��ʾ)�ĵ�������NaOH��Һ��Ӧ�� ����(Na2RO3)��������
����(Na2RO3)��������
��ش��������⣺
(1)д�������ʵĻ�ѧʽ��A__________��B__________��C__________��D__________��E__________��
(2)д����Ӧ�ٵĻ�ѧ����ʽ��____________________________________________��
�÷�Ӧ���������뻹ԭ�������ʵ���֮��Ϊ________��
(3)д����Ӧ�ܵ����ӷ���ʽ��__________________________________________��
(4)д����Ӧ�ݵ����ӷ���ʽ��___________________________________________��
(5)H2CO3������ǿ��E�ģ��������ӷ���ʽ����֤����
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��AԪ�ص�һ�ֵ�����һ����Ҫ�İ뵼����ϣ���AԪ�ص�һ�ֻ�����C��������������ܵ��ִ�ͨѶ���ϡ������ά��C���ռӦ���ɺ�AԪ�صĻ�����D��
(1)��Ԫ�����ڱ��У�Aλ��________�壬��Aͬ�嵫���ԭ��������AС��Ԫ��B��ԭ�ӽṹʾ��ͼΪ________��A��B��ԭ�ӵĵ��Ӳ�ṹ�ϵ���ͬ����________________________________________________________________________
________________________________________________________________________��
(2)����C������ѧ��Ӧ������________(д����)����Ӧ�Ļ�ѧ����ʽ��________________________________________________________________________��
(3)��C�봿���ϸ�������ʱҲ������ѧ��Ӧ����D��ͬʱ������B�����������E����ȫ����E��ȫ����D��������ˮ�л�Ϻ��ַ�����ѧ��Ӧ���ɺ�A�Ļ�����F��
��д������D��F�Ļ�ѧ��Ӧ����ʽ��_____________________________________
________________________________________________________________________��
��Ҫ����������ۻ������������п�ѡ�õ���________��
A����ͨ�������� B��ʯӢ��������
C������������ D��������
(4)100 g C��ʯ��ʯ�Ļ�����ַ�Ӧ�����ɵ������ڱ�״���µ����Ϊ11.2 L,
100 g�������ʯ��ʯ������������____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ˮ�ﵽƽ����������������䣬ֻ�ı�ijһ����������������ȷ����(����)
A����ͨ������������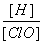 ��С
��С
B��ͨ������SO2����ҺƯ������ǿ
C��������������NaOH��һ����[Na��]��[Cl��]��[ClO��]
D����������ˮ��ˮ�ĵ���ƽ��������Ӧ�����ƶ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com