
��12�֣���ͼ��ʵ������ȡ����������ʵ��װ�á���ش�
��1����M�Թ��м����Ƭ�������� ��
�Թ�N��ʢ�ŵ���Һ��_________________ ��
����Һ������Ϊ�к����ᣬ���ջӷ������Ҵ��� ��
�������� Һ���϶�������Һ���µ�ԭ���� ��
��2���ڴ��Թ�������һ���������Ҵ���Ũ���������
���Һʱ�������Լ���˳���� ��
��3��Ũ����������� ��
��4����Ӧ�������Թ�N�ڵ�Һ��ֳ����㣬��Ӧ���ɵ����������� �㣨��д���ϡ����¡�����������N�е�Һ��������Ҫ�õ��IJ����������ձ��� ��
��5��M�Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��6���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ���
�ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ����������
����Ӧ�Ѵﵽ��ѧƽ��״̬��������������������(�����)��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
��1����ֹ���С�����̼������Һ�����������������ܽ�ȣ����ڷֲ㡣��ֹ������
��2���ȼ����Ҵ����ڻ�������Ũ���ᣬ��ȴ���ڼ������ᡣ
��3����������ˮ����
��4���ϣ���Һ©����
![]() ��5��CH3COOH + C2H5OH CH3COOC2H5 + H2O
��5��CH3COOH + C2H5OH CH3COOC2H5 + H2O
��6����
����:���������������Ʊ���
��1��������Ӧ��Ҫ���ȣ�Ϊ�˷�ֹҺ�����ʱ������������Ҫ�������Ƭ�Է�ֹ���С�������Ҵ����ӷ����������ɵ����������к���������Ҵ������뱥��̼����һ������������Ҵ����к����ᣬ��һ������Խ��������������ܽ�ȣ������ڷֲ�������������Ҵ���ˮ���ܣ����Բ���ֱ�Ӳ�����Һ�У��Է�ֹ������
��2��Ũ��������ˮ�ų��������ȣ���Ũ������ܶȴ���ˮ�ģ�Ϊ�˷�ֹ������Ҵ��Ļӷ���Ӧ���ȼ����Ҵ����ڻ�������Ũ���ᣬ��ȴ���ڼ������ᡣ
��3����Ϊ�ǿ��淴Ӧ������Ũ����������������⣬������ˮ�������ã�ʹ��Ӧ���������������������ķ����ƶ���
![]() ��4�������������ܶȱ�ˮ��С����������ˮ���������ϲ㣬ͨ����Һ©������ʵ�ַ��롣
��4�������������ܶȱ�ˮ��С����������ˮ���������ϲ㣬ͨ����Һ©������ʵ�ַ��롣
��5����Ӧ����ʽΪCH3COOH + C2H5OH CH3COOC2H5 + H2O��
��6���٢��з�Ӧ���ʵķ�������ͬ�ģ����κ�����º�������ڷ�Ӧ���ʵķ������෴�ģ�����������֮������Ӧ�Ļ�ѧ������֮�ȣ���ȷ��


 �������Ӧ���⼯ѵϵ�д�
�������Ӧ���⼯ѵϵ�д� �ۺ��Բ�ϵ�д�
�ۺ��Բ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2011-2012����Ϸ���ػ�����ѧ��һ��ѧ�ڵ������¿����ģ���ѧ������������ ���ͣ�ʵ����
��12�֣���ͼ��ʵ������ȡ����������ʵ��װ�á���ش�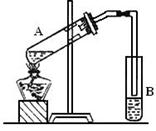
��1����M�Թ��м����Ƭ�������� ��
�Թ�N��ʢ�ŵ���Һ��_________________ ��
����Һ������Ϊ�к����ᣬ���ջӷ������Ҵ��� ��
�������� Һ���϶�������Һ���µ�ԭ���� ��
��2���ڴ��Թ�������һ���������Ҵ���Ũ���������
���Һʱ�������Լ���˳���� ��
��3��Ũ����������� ��
��4����Ӧ�������Թ�N�ڵ�Һ��ֳ����㣬��Ӧ���ɵ����������� �㣨��д���ϡ����¡�����������N�е�Һ��������Ҫ�õ��IJ����������ձ��� ��
��5��M�Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��6���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ���
�ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ����������
����Ӧ�Ѵﵽ��ѧƽ��״̬��������������������(�����)��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012����Ϸ���ػ�����ѧ��һ��ѧ�ڵ������¿����ģ���ѧ���������棩 ���ͣ�ʵ����
��12�֣���ͼ��ʵ������ȡ����������ʵ��װ�á���ش�

��1����M�Թ��м����Ƭ�������� ��
�Թ�N��ʢ�ŵ���Һ��_________________ ��
����Һ������Ϊ�к����ᣬ���ջӷ������Ҵ��� ��
�������� Һ���϶�������Һ���µ�ԭ���� ��
��2���ڴ��Թ�������һ���������Ҵ���Ũ���������
���Һʱ�������Լ���˳���� ��
��3��Ũ����������� ��
��4����Ӧ�������Թ�N�ڵ�Һ��ֳ����㣬��Ӧ���ɵ����������� �㣨��д���ϡ����¡�����������N�е�Һ��������Ҫ�õ��IJ����������ձ��� ��
��5��M�Թ��з�����Ӧ�Ļ�ѧ����ʽ ��
��6���������������ķ�Ӧ�ǿ��淴Ӧ����Ӧ�ﲻ����ȫ����������Ӧһ��ʱ���
�ʹﵽ�˸÷�Ӧ���ȣ�Ҳ���ﵽ��ѧƽ��״̬������������˵���Ҵ����������
����Ӧ�Ѵﵽ��ѧƽ��״̬��������������������(�����)��
�ٵ�λʱ�������1mol����������ͬʱ����1molˮ
�ڵ�λʱ�������1mol����������ͬʱ����1mol����
�۵�λʱ�������1mol�Ҵ���ͬʱ����1mol����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʵ������ȡ����������װ�á���ش��������⣺

(1)C��Ҫ����3mL�Ҵ���2mLŨ�����2mL���ᣬŨ�����ڷ�Ӧ����������� ��
(2)��C���Թ��л�Ӧ�����������Ƭ���������� ��
(3) C��������Ӧ�Ļ�ѧ����ʽΪ ��
(4)D���Թ���Ԥ��װ����DZ���Na2CO3��Һ�������������
����Ӧ������D�Թ��п������Կ�����ɫҺ���Ϊ���㣬���ϲ�Һ���� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com