
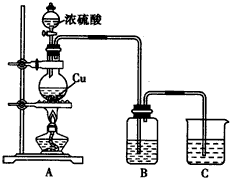
��ѧʵ��С����ѧϰ��ѧ��Դ���ȼҵ���֪ʶ����ʵ���� ����ʵ����֤�����������װ������ͼ��ʾװ�ã���֪Ϊʯī�缫��Ϊ���缫��Ϊ���缫���ѳ�ȥ��������Ĥ����Ϊ���ʯī�缫���ձ�������������ʳ��ˮ�����з�̪�������õ��ߺ�����ָ�뷢������ƫת�������ж���ȷ����
A������������
B��һ��ʱ����缫������Һ���
C���缫�缫��ӦʽΪ��2Cl��2e-=Cl2��
D�������һ��ʱ�䣬B�г��ִ�����ɫ����ʱ��ֹͣ
ʵ�飬�ٽ�A����Һ����B�л�ϣ������
����ȫ����ʧ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʵ�ֻ�ѧʵ����ɫ����Ҫ����Ч�ؼ�����Ⱦ���ɰѻ�ѧʵ����Ƴ��ͻ���ij�о���ѧϰС���ѧ�������в�����һ��ʵ�飺��һ���³İ�ֽ�IJ���Ƭ�IJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1 mol/L��KBr��KI����������Һ����NaOH������̪����FeCl2����KSCN����Һ��1�Σ�ÿ��Һ�α˴˷ֿ���Χ�ɰ뾶С�ڱ������Բ��(����ͼ��ʾ)����Բ�Ĵ�����2��֥������С��KMnO4���壬��KMnO4����μ�һ��Ũ���ᣬ�������ñ�����Ǻá�
(1)��a���۲쵽��ʵ������_____________________________________��
��c���۲쵽��ʵ������______________________________________��
(2)e����Ӧ�����ӷ���ʽΪ___________________________________��
�÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ__________________________��
(3) d����Ӧ������������Ϊ___ _______________��
��״���£�����0.112 L Cl2��NaOH��Һ���պ�ת�Ƶ���Ϊ________mol��
(4)ͨ����ʵ���ܷ�Ƚ�Cl2��FeCl3��KMnO4�������������Ե�ǿ����________����ܡ����ܡ��������ܣ�����������ǿ������˳����____________ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011�츣��ʡ�����а��أ��У�������ѧ������������ѧ�� ���ͣ������
ʵ�ֻ�ѧʵ����ɫ����Ҫ����Ч�ؼ�����Ⱦ���ɰѻ�ѧʵ����Ƴ��ͻ���ij�о���ѧϰС���ѧ�������в�����һ��ʵ�飺��һ���³İ�ֽ�IJ���Ƭ�IJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1 mol/L��KBr��KI����������Һ����NaOH������̪����FeCl2����KSCN����Һ��1�Σ�ÿ��Һ�α˴˷ֿ���Χ�ɰ뾶С�ڱ������Բ��(����ͼ��ʾ)����Բ�Ĵ�����2��֥������С��KMnO4���壬��KMnO4����μ�һ��Ũ���ᣬ�������ñ�����Ǻá�
(1)��a���۲쵽��ʵ������_____________________________________��
��c���۲쵽��ʵ������______________________________________��
(2)e����Ӧ�����ӷ���ʽΪ___________________________________��
�÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ__________________________��
(3) d����Ӧ������������Ϊ___ _______________��
��״���£�����0.112 L Cl2��NaOH��Һ���պ�ת�Ƶ���Ϊ________mol��
(4)ͨ����ʵ���ܷ�Ƚ�Cl2��FeCl3��KMnO4�������������Ե�ǿ����________����ܡ����ܡ��������ܣ�����������ǿ������˳����____________ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫ʡ�ع��и����ڶ���ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�ʵ����
ij��ѧ��ȤС����ѧϰ�������ε�ijЩ���ʺ���;���У�����������ʵ��̽����
��ʵ��һ������(NH4)2Fe(SO4)2��6H2O��Һ�к��е������ӡ�
��1�����±���д�����������ʵ������
��ѡ�Լ���������ˮ��1mol/LH2SO4��Һ��0.1mol/L KSCN��Һ��NaOHŨ��Һ��Ũ���ᡢ1mol/LNa2CO3��Һ
|
�� �� �� �� |
ʵ������ |
�� �� |
|
����I��ȡ��������Һ���Թ��У� �� |
|
��Һ�к���Fe2+ |
|
����II��ȡ��������Һ���Թ��У� �� |
|
��Һ�к���NH4�� |
��ʵ�������KHSO4��ȡH2O2����������������
�������ϵ�֪����ҵ���õ��KHSO4������Һ����ȡһ��Ũ�ȵ�H2O2����ȤС���������ʵ��ⶨH2O2��������������֪��2MnO4��+5H2O2+6H��= 2Mn2��+8H2O+5O2��
�����٣�ȡ5.00 mL H2O2��Һ(�ܶ�Ϊ1.00 g/mL)������ƿ�м�ˮϡ�ͣ��ټ�ϡ�����ữ��
�����ڣ���0.1000 mol/L KMnO4��Һ�ζ���
�����ۣ���ͬ�������ζ����Ĵ�����KMnO4��Һ������ֱ�Ϊ20.00 mL��19.98 mL��20.02 mL��22.00 mL��
�ش��������⣺
��2���������У���ʼ����KMnO4��Һʱ��Ӧ���ʺ���������KMnO4��Һ���뷴Ӧ���������ӿ죬���п��ܵ�ԭ��______________________��
��3���ζ�ʱʢװKMnO4��ҺӦѡȡ�������� ������ţ���
A��50mL��ʽ�ζ��ܡ� B��50mL��ʽ�ζ��� ��
C��25mL��ʽ�ζ��ܡ� D��25mL��ʽ�ζ���
��4��������ʵ���У����в�������ɲⶨ���ƫ�ߵ��� ������ţ���
A����ƿ�ô���Һ��ϴ
B����ȡ H2O2��Һ�ĵζ���������ˮϴ����δ��H2O2��Һ��ϴ
C���ζ��ٶȹ��죬��δҡ�ȣ�ֹͣ�ζ������Ϻ�ɫ��ȥ
D���ζ�ǰ����ʱƽ�ӣ��ζ��յ����ʱ����
��5���������ݣ�����H2O2��Һ�����ʵ������ٷֺ���Ϊ___________��������λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ�����а��أ��У�������ѧ������������ѧ�� ���ͣ������
ʵ�ֻ�ѧʵ����ɫ����Ҫ����Ч�ؼ�����Ⱦ���ɰѻ�ѧʵ����Ƴ��ͻ���ij�о���ѧϰС���ѧ�������в�����һ��ʵ�飺��һ���³İ�ֽ�IJ���Ƭ�IJ�ͬλ�÷ֱ�μ�Ũ��Ϊ0.1 mol/L��KBr��KI����������Һ����NaOH������̪����FeCl2����KSCN����Һ��1�Σ�ÿ��Һ�α˴˷ֿ���Χ�ɰ뾶С�ڱ������Բ��(����ͼ��ʾ)����Բ�Ĵ�����2��֥������С��KMnO4���壬��KMnO4����μ�һ��Ũ���ᣬ�������ñ�����Ǻá�
(1)��a���۲쵽��ʵ������_____________________________________��
��c���۲쵽��ʵ������______________________________________��
(2)e����Ӧ�����ӷ���ʽΪ___________________________________��
�÷�Ӧ���������ͻ�ԭ�������ʵ���֮��Ϊ__________________________��
(3) d����Ӧ������������Ϊ___ _______________��
��״���£�����0.112 L Cl2��NaOH��Һ���պ�ת�Ƶ���Ϊ________mol��
(4)ͨ����ʵ���ܷ�Ƚ�Cl2��FeCl3��KMnO4�������������Ե�ǿ����________����ܡ����ܡ��������ܣ�����������ǿ������˳����____________ ___��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ר���� ���ͣ�ʵ����


�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com