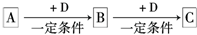ŅŃÖŖA”¢B”¢CŹĒ֊ѧ»ÆѧµÄ³£¼ūĪļÖŹ£¬ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀÓŠČēĻĀ×Ŗ»Æ¹ŲĻµ£ŗ
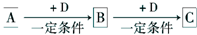
£Ø1£©ČōAÄÜŹ¹ŹŖČóµÄŗģÉ«ŹÆČļŹŌÖ½±äĄ¶£»CĪŖŗģ×ŲÉ«ĘųĢ壮ŌņA×Ŗ»ÆĪŖB·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
£®
£Ø2£©ČōDŹĒ¾ßÓŠŃõ»ÆŠŌµÄµ„ÖŹ£¬AŌŖĖŲĪŖµŚČżÖÜĘŚµÄ½šŹōŌŖĖŲ£¬ŌņCµÄµē×ÓŹ½ĪŖ
£®
£Ø3£©ČōDŹĒÉś»īÖŠ³£¼ūµÄ¹ż¶É½šŹō£¬AŹĒ»ĘĀĢÉ«ĘųĢ壬ŌņB”śCµÄĄė×Ó·½³ĢŹ½ĪŖ
2Fe3++Fe=3Fe2+
2Fe3++Fe=3Fe2+
£®
£Ø4£©ČōDŹĒŅ»ÖÖ³£¼ūµÄĪĀŹŅĘųĢ壻AŹĒŅ»ÖÖĒæµē½āÖŹĒŅŌŚĖ®ČÜŅŗÖŠµēĄė³öµÄŅõ”¢ŃōĄė×Ó¾łŗ¬ÓŠ10øöµē×Ó£®ŌņB×Ŗ»ÆĪŖCµÄĄė×Ó·½³ĢŹ½ĪŖ
CO32-+CO2+H2O=HCO3-
CO32-+CO2+H2O=HCO3-
£®
£Ø5£©ČōAĪŖ×ī¼ņµ„µÄÓŠ»śĪļ£¬DŹĒ¾ßÓŠŃõ»ÆŠŌµÄµ„ÖŹ£®25”ę”¢101kPaŹ±£¬3.2g AĶźČ«Č¼ÉÕÉś³ÉĪČ¶ØµÄŃõ»ÆĪļŹ±·Å³ö178kJµÄČČĮ森ĒėŠ“³ö±ķŹ¾ĪļÖŹAµÄČ¼ÉÕČȵÄČČ»Æѧ·½³ĢŹ½
CH4£Øg£©+2O2£Øg£©=CO2£Øg£©+2H2O£Øl £©”÷H=-890kJ/mol
CH4£Øg£©+2O2£Øg£©=CO2£Øg£©+2H2O£Øl £©”÷H=-890kJ/mol
£®
£Ø6£©ČōAµÄĢå»ż·ÖŹżĪŖ75%µÄČÜŅŗæÉÓĆ×÷Ņ½ĮĘĻū¶¾¼Į£»BÓėŠĀÖĘĒāŃõ»ÆĶ¹²ČČ£¬ÓŠŗģÉ«³ĮµķÉś³É£®ŌņAÉś³ÉBµÄ»Æѧ·½³ĢĪŖ
£®






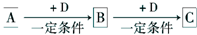
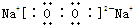
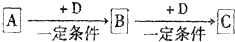 ŅŃÖŖA”¢B”¢CŹĒ֊ѧ»ÆѧµÄ³£¼ūĪļÖŹ£¬ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀÓŠČēĶ¼ĖłŹ¾×Ŗ»Æ¹ŲĻµ£ŗ
ŅŃÖŖA”¢B”¢CŹĒ֊ѧ»ÆѧµÄ³£¼ūĪļÖŹ£¬ĖüĆĒŌŚŅ»¶ØĢõ¼žĻĀÓŠČēĶ¼ĖłŹ¾×Ŗ»Æ¹ŲĻµ£ŗ