
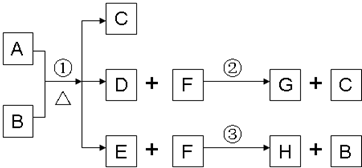
A��GΪ��ѧ�����Ļ��������֮������ͼ
��ʾ��ת����ϵ����Ӧ���������ֲ�������ȥ����AΪ��ɫ
��ĩ����H��C��O��Cu����Ԫ�ء�������DΪ��ɫ��ζ
���壬BΪ��ɫ��ĩ��E�ܷ���������Ӧ����ش�
��1��D��G��Ӧ�Ļ�ѧ����ʽΪ_____________________________________��

��2��F��һ�����еĹ����ŵ�����Ϊ_______________________��
��3��ij����С��ͬѧ���������ʵ��װ�ã�ͨ���ⶨijЩװ�����Լ��������仯��̽��A�и�Ԫ�ص�������ϵ��
�� Ϊʹ����ȷ�����貹��װ�ã������ڷ����ڻ��װ��ͼ��д���Լ����ƣ�
�� ��װ���й��������Ŀ����_________________________________________________________��
��װ����ҩƷ������Ϊ_______________________________��ʵ��ʱ����ҩƷδ�����Ա仯��֤��_______________________________________________________________________________��
�� ����ж�A����ȫ�ֽ⣿____________________________________________________________��
�� ����ȷ�IJⶨ�ó��������ݣ�A���Ⱥ���ȫ�ֽ⣬������8.0 g��Ϊ6.0 g��װ��������0.90 g��д��A�Ļ�ѧʽ����ʾΪ��ʽ�Σ���__________________________________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ����
��֪A��B��D��E��Ϊ��ѧ��ѧ�������ʻ������֮��Ĺ�ϵ��ͼ��ʾ�����ֲ�����ȥ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| ||
| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
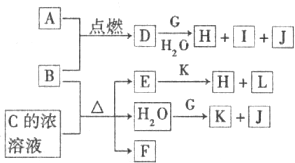
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
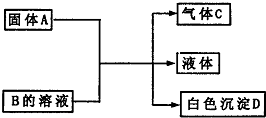 A��B��C��DΪ��ѧ��ѧ���������ʣ����Ǽ�ķ�Ӧ��ϵ��ͼ��ʾ��
A��B��C��DΪ��ѧ��ѧ���������ʣ����Ǽ�ķ�Ӧ��ϵ��ͼ��ʾ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ������人�������������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ������
������G�Ǻϳɿ���ҩ���������м��壬���Ľṹ��ʽΪ��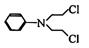
��������ѧ�����ļ��л���Ϊ��Ҫԭ�Ϻϳɣ���ϳ���·���£�
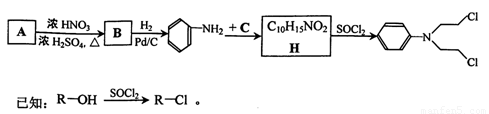
��1��D��A��ɵ�Ԫ����ͬ��ԭ�Ӹ�����Ҳ��ͬ����Ҫ��д������������D�Ľṹ��ʽ��
�ٷ��ӵĿռ乹��Ϊֱ���͵ķ��� ��
�ڷ��������е�̼ԭ�Ӿ�Ϊsp3�ӻ��ķ��� ��
��NMRͼ������5���壬�������֮��Ϊ2:1:2:2:1�ķ��� ��
��2��B��һ�ȴ���������ͬ���칹�壬B������������һ�������·�Ӧ���ɱ����л���Y����Y���ϵ�һ�ȴ����ͬ���칹���� �֣�
��3����֪�����봼��һ���������ܷ������Ӽ����ˮ��Ӧ����д������������C�����ʵ���֮��Ϊ1:3��Ӧ���ɵIJ���Ľṹ��ʽ �����л����к��еĹ����ŵ������� ��
��4��д���ڼ�������Ũ������������£�H���Ҷ��������ʵ�����Ϊ1:1��Ӧ�Ļ�ѧ����ʽ ��
�÷�Ӧ�������� ��
��5��������H��һ��ͬ���칹��X�������Ȼ�����Һ������ɫ��Ӧ��������Ũ��ˮ��Ӧ��1 mol X�������3 mol Br2��д��X�Ľṹ��ʽ ����Ҫ��д�����֣�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com