
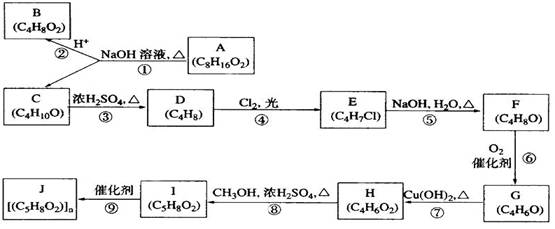
 ( l ) B��C��Ϊ��֧�����л������B �Ľṹ��ʽ______________________��C��Ũ���������¼��ȷ�Ӧֻ������һ��ϩ��D , D�Ľṹ��ʽΪ��__________________��I�Ľṹ��ʽ��______________________��
( l ) B��C��Ϊ��֧�����л������B �Ľṹ��ʽ______________________��C��Ũ���������¼��ȷ�Ӧֻ������һ��ϩ��D , D�Ľṹ��ʽΪ��__________________��I�Ľṹ��ʽ��______________________��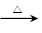 CH2=C(CH3)CH2OH + NaCl
CH2=C(CH3)CH2OH + NaCl


 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
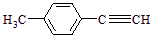
 R��CH2CHO + R��OH
R��CH2CHO + R��OH 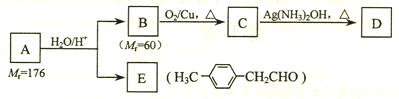
 ����һ��·�����£�
����һ��·�����£�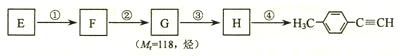
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
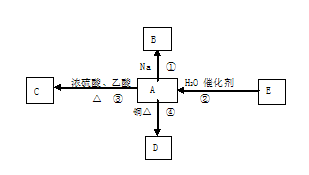
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
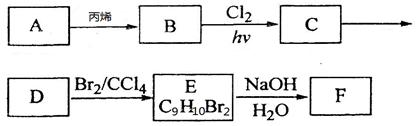
 _��
_���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
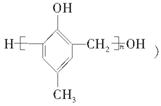 �ĺϳ�·�����£�
�ĺϳ�·�����£�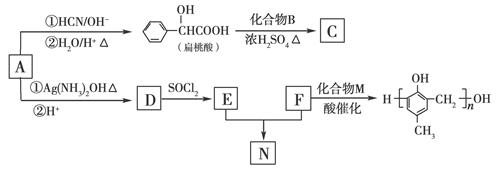

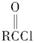 RCOOR�䡡(R��R���������)
RCOOR�䡡(R��R���������)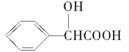 )�ж���ͬ���칹�塣���ڼ������Һ����ǻ���ͬ���칹�干��________�֣�д������һ�ֺ��Ǽ�(��CH2��)��ͬ���칹��Ľṹ��ʽ________��
)�ж���ͬ���칹�塣���ڼ������Һ����ǻ���ͬ���칹�干��________�֣�д������һ�ֺ��Ǽ�(��CH2��)��ͬ���칹��Ľṹ��ʽ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
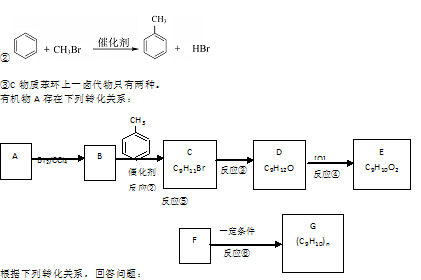
 �Ļ�ѧ����ʽ�� ��
�Ļ�ѧ����ʽ�� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
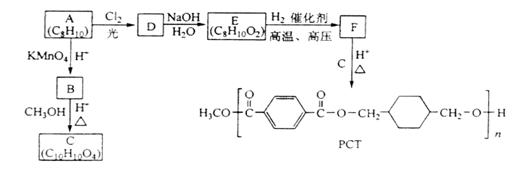
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com