
| A����101 kPa��25 ��ʱ���к���Ϊ57.3 kJ/mol�����ʾBa(OH)2��ϡ��Һ������ϡ���ᷴӦ���Ȼ�ѧ����ʽΪ�� Ba(OH)2(aq)��H2SO4(aq)��BaSO4(s)��2H2O(l) ��H����114.6 kJ/mol |
| B����101 kPa��25 ��ʱ��2 g H2��ȫȼ������Һ̬ˮ���ų�285.8 kJ��������������ȼ�յ��Ȼ�ѧ����ʽ��ʾΪ��2H2(g)��O2(g)��2H2O(l) ��H����285.8 kJ/mol |
| C��pH=11�İ�ˮ��pH=3������������ϣ�����NH4Clˮ���ʹ��Һ������ |
| D������ͬ����£�ǿ�������Һ�ĵ����Ա���ͬŨ�����������Һ�ĵ�����ǿ |

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 4NO (g)+ 6H2O(g)
4NO (g)+ 6H2O(g)
| A����ͼ��֪���÷�Ӧ������Ӧ�����ȷ�Ӧ |
| B�������¶ȿ������NH3��ת���� |
| C����Ӧ��ϵ�м�����������ӷ�Ӧ�� |
| D����ƽ������ͨ��ˮ��һ������������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
|
 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣�
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ�����ݻ�Ϊ2 L���ܱ������г���10 mol N2��5 mol O2���ﵽƽ���NO�����ʵ���Ϊ2 mol����T ��ʱ�÷�Ӧ��ƽ�ⳣ��K= �� ��������������С�������λ���֣� 
 N2(g)+O2(g)Ϊ(����ȡ����ȡ�) �� ��Ӧ��
N2(g)+O2(g)Ϊ(����ȡ����ȡ�) �� ��Ӧ�� N2(g)+O2(g) �Ѵﵽƽ����ǣ�����ţ��� ��
N2(g)+O2(g) �Ѵﵽƽ����ǣ�����ţ��� ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��H2O��g����H2��g����1/2O2��g����H����285.8kJ��mol��1 |
| B��2H2��g����O2��g����2H2O��l����H����517.6kJ��mol��1 |
| C��H2��g����1/2 O2��g����H2O��g����H����285.8kJ��mol��1- |
| D��2H2��g����O2��g����2H2O��l����H����517.6J��mol��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��˸�ͬѧ�ó����ۡ�ú̿ȼ��ʱ������ˮ����ʹú̿ȼ�շų������������������֪ú̿��ȼ����Ϊ��393.15 kJ/mol��������ȼ����Ϊ��242 kJ/mol��һ����̼��ȼ����Ϊ��283 kJ/mol��
��˸�ͬѧ�ó����ۡ�ú̿ȼ��ʱ������ˮ����ʹú̿ȼ�շų������������������֪ú̿��ȼ����Ϊ��393.15 kJ/mol��������ȼ����Ϊ��242 kJ/mol��һ����̼��ȼ����Ϊ��283 kJ/mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 N2��
N2�� 2N
2N 2N��
2N�� NH��
NH�� NH2��
NH2�� NH3��
NH3�� NH3
NH3
 +
+ ��ij�¶��������ӻ�����Ϊ10-30����Һ���е�pNH4��ˮ�е�pH���ƣ�����¶���Һ����pNH4=_________________��
��ij�¶��������ӻ�����Ϊ10-30����Һ���е�pNH4��ˮ�е�pH���ƣ�����¶���Һ����pNH4=_________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ӧ��������������������������ʱ���÷�Ӧһ�����ܷ��� |
| B��ǿ���ǿ�Ӧ�ų������������к��� |
C����ʯī�Ƚ��ʯ�ȶ���֪�� |
D���� �� �� ʱ�� ʱ�� ��ȫȼ��������̬ˮ���ų� ��ȫȼ��������̬ˮ���ų� ��������������ȼ����Ϊ241.8 ��������������ȼ����Ϊ241.8 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


 ��
�� ������˵����ȷ���ǣ� ��
������˵����ȷ���ǣ� ��A����Ȳ��ȼ����Ϊ |
B����ת�� ���ӣ������� ���ӣ������� |
C��������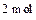 Һ̬ˮ���� Һ̬ˮ���� |
D�����γ� ̼�����õ���ʱ����ų�������Ϊ ̼�����õ���ʱ����ų�������Ϊ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com