
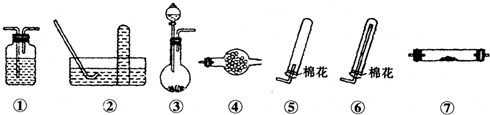
| ���� | �۵�/�� | �е�� | ��ѧ���� | ||||
| S | 112.8 | 444.6 | �� | ||||
| S2Cl2 | -77 | 137 | ��ˮ����HCl��SO2��S��300��������ȫ�ֽ⣻ S2Cl2+Cl2
|
| ||
| ||
| ||



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ���� | �۵�/�� | �е�� | ��ѧ���� | ||||
| S | 112.8 | 444.6 | �� | ||||
| S2Cl2 | -77 | 137 | ��ˮ����HCl��SO2��S��300��������ȫ�ֽ⣻ S2Cl2+Cl2
|
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(11��)ij̽��С����ʵ��������������(��Ҫ�ɷ�ΪAl2O3��������Fe2O3��SiO2)��ȡ���������ش��������⣺
(1)��ʵ��������1 mol��L��1��NaOH��Һ480 mL�����Ƹ���Һ��������������������ƽ(����)����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ�������_______________________��
�����ղ������õ����������е�һ�֣���������________��
(2)д��������з�����Ӧ�����ӷ���ʽ____________________________________
________________________________________________________________________��
(3)��������ϴ����β���_______________________________________________
________________________________________________________________________��
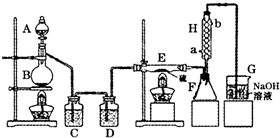
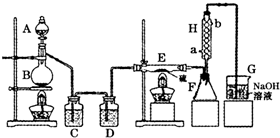
(4)��ͬѧ��ʵ������������װ���Ʊ�CO2���壬��ͨ����ҺB����ȡAl(OH)3ʱ�����û�в���Ԥ������
��ͬѧ������Ϊ����ͬѧͨ��CO2�����ǵ���ʵ��ʧ�ܵ�ԭ��֮һ������Ϊ�ҵķ����Ƿ������________�����������������ӷ���ʽ������ԭ��________________________________________________________��
(������Ϊ���������ÿղ�����)
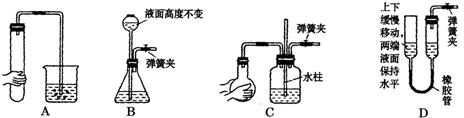
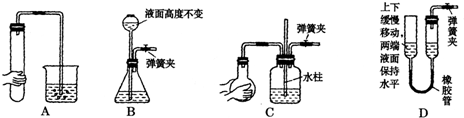
��ͬѧ������Ϊ����ͬѧͨ���CO2�к���HCl���壬Ҳ�ǵ���ʵ��ʧ�ܵ�ԭ����ʵ��������ijװ�ÿɽ��������⡣�������ͬѧ������װ��ͼ����ע���Լ����ơ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���ӿ��ﵽ�������ϡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ���ѡ��
(11��)ij̽��С����ʵ��������������(��Ҫ�ɷ�ΪAl2O3��������Fe2O3��SiO2)��ȡ���������ش��������⣺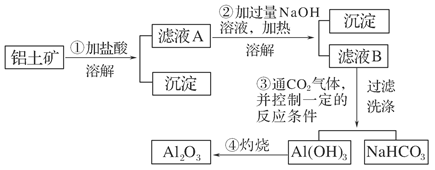
(1)��ʵ��������1 mol��L��1��NaOH��Һ480 mL�����Ƹ���Һ��������������������ƽ(����)����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ�������_______________________��
�����ղ������õ����������е�һ�֣���������________��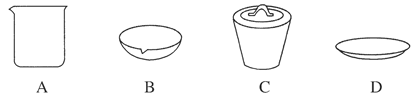
(2)д��������з�����Ӧ�����ӷ���ʽ____________________________________
________________________________________________________________________��
(3)��������ϴ����β���_______________________________________________
________________________________________________________________________��
(4)��ͬѧ��ʵ������������װ���Ʊ�CO2���壬��ͨ����ҺB����ȡAl(OH)3ʱ�����û�в���Ԥ������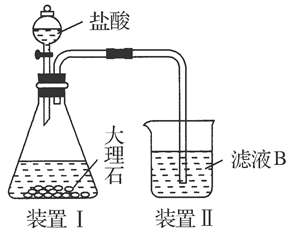
��ͬѧ������Ϊ����ͬѧͨ��CO2�����ǵ���ʵ��ʧ�ܵ�ԭ��֮һ������Ϊ�ҵķ����Ƿ������________�����������������ӷ���ʽ������ԭ��________________________________________________________��
(������Ϊ���������ÿղ�����)
��ͬѧ������Ϊ����ͬѧͨ���CO2�к���HCl���壬Ҳ�ǵ���ʵ��ʧ�ܵ�ԭ����ʵ��������ijװ�ÿɽ��������⡣�������ͬѧ������װ��ͼ����ע���Լ����ơ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ���ӿ��ﵽ�������ϡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ѡ����
(11��)ij̽��С����ʵ��������������(��Ҫ�ɷ�ΪAl2O3��������Fe2O3��SiO2)��ȡ���������ش��������⣺
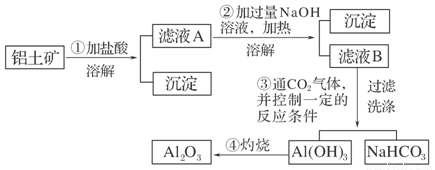
(1)��ʵ��������1 mol��L��1��NaOH��Һ480 mL�����Ƹ���Һ��������������������ƽ(����)����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ�������_______________________��
�����ղ������õ����������е�һ�֣���������________��
(2)д��������з�����Ӧ�����ӷ���ʽ____________________________________
________________________________________________________________________��
(3)��������ϴ����β���_______________________________________________
________________________________________________________________________��
(4)��ͬѧ��ʵ������������װ���Ʊ�CO2���壬��ͨ����ҺB����ȡAl(OH)3ʱ�����û�в���Ԥ������
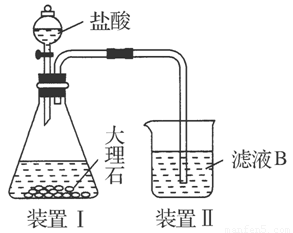
��ͬѧ������Ϊ����ͬѧͨ��CO2�����ǵ���ʵ��ʧ�ܵ�ԭ��֮һ������Ϊ�ҵķ����Ƿ������________�����������������ӷ���ʽ������ԭ��________________________________________________________��
(������Ϊ���������ÿղ�����)
��ͬѧ������Ϊ����ͬѧͨ���CO2�к���HCl���壬Ҳ�ǵ���ʵ��ʧ�ܵ�ԭ����ʵ��������ijװ�ÿɽ��������⡣�������ͬѧ������װ��ͼ����ע���Լ����ơ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ������ʡ����1���¿������ۣ���ѧ���� ���ͣ�ʵ����
ij̽��С����ʵ������
����������Ҫ�ɷ�ΪAl2O3��������Fe2O3��
SiO2����ȡ���������ش��������⣺
��1����ʵ��������1mol��L ��NaOH��Һ480mL�����Ƹ���Һ��������������������ƽ�����룩����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ������� ��
��NaOH��Һ480mL�����Ƹ���Һ��������������������ƽ�����룩����ͷ�ιܡ�ҩ�ס�����������ȱ�ٵ������� ��
�����ղ������õ����������е�һ�֣��������� ��
��2��д��������з�����Ӧ�����ӷ���ʽ ��
��3����������ϴ����β��� ��
��4����ͬѧ��ʵ������������װ���Ʊ�CO2���壬��ͨ����ҺB���Ʊ�Al��OH��3ʱ�����û�в���Ԥ������
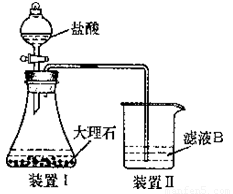
��ͬѧ������Ϊ����ͬѧͨ��CO2�����ǵ���ʵ��ʧ�ܵ�ԭ��֮һ������Ϊ�ҵķ����Ƿ����? ��
���������������ӷ���ʽ������ԭ�� ��
��������Ϊ���������ÿղ�����
��ͬѧ������Ϊ����ͬѧͨ�˵�CO2�к���HCl���壬Ҳ�ǵ���ʵ��ʧ�ܵ�ԭ����ʵ��������ijװ�ÿɽ��������⡣�������ͬѧ������װ��ͼ����ע���Լ����ơ�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com