
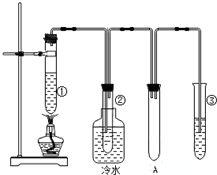
 ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ�����������Ѻ��ԣ������Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ��
ʯ���ͣ�17��̼ԭ�����ϵ�Һ̬���������ķֽ�ʵ��װ����ͼ��ʾ�����������Ѻ��ԣ������Թܢ��м���ʯ���ͺ�����������ʯ���ֽ⣩���Թܢڷ�����ˮ�У��Թܢ��м�����ˮ�� B��
B��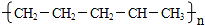
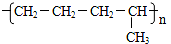 D��
D��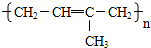
���� ��1���ѻ����к���ϩ����������ˮ�����ӳɷ�Ӧ��A���ڷ�ֹ������
��2��C4H10��CH4+CH2=CHCH3��C4H10��C2H6+CH2=CH2�������в����ͼ���˫��������������˫�����Ļ������״�ͷ��ӻ�����ڴ�����������������������������£���ӳ��γ��µĹ��ۼ���������ӵķ�Ӧ���ǼӾ۷�Ӧ��������ͬ����֮��ӳɾۺ�Ҳ���Dz�ͬ���ʼ�ӳɾۺϣ�
��3���Թܢ���CH2=CH-CH3��CH2=CH2������ˮ�����ӳɷ�Ӧ��
��4��ʯ���ڴ��������������£����ȿ����ѻ�Ϊ��������̬�������Ͳ�������������Һ̬�ı������Ͳ���������
��� �⣺��1���ѽ�����CH2=CH-CH3��CH2=CH2������ˮ�����ӳɷ�Ӧ��ʹ��ѹ���ͣ�����������װ��A�������Ƿ�ֹ�Թܢ���Һ�嵹�����Թܢ��У�
�ʴ�Ϊ����ֹ�Թܢ���Һ�嵹�����Թܢ��У���������ȫƿ����
��2������������ѽⷽʽΪ��C4H10��CH4+CH2=CHCH3��C4H10��C2H6+CH2=CH2���������ϩ�ͱ�ϩ֮�䷢���Ӿ۷�Ӧ������̼ԭ��֮�������˳������� ��
��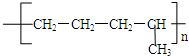 ���ֽṹ��ʽ���ֱ�ΪA��C�ṹ��
���ֽṹ��ʽ���ֱ�ΪA��C�ṹ��
�ʴ�Ϊ��CH2=CH-CH3��CH2=CH2���Ӿ۷�Ӧ��AC��
��3��CH2=CH-CH3��CH2=CH2������ˮ�����ӳɷ�Ӧ����CH2=CH2+Br2��CH2BrCH2Br����дCH3CH=CH2+Br2��CH3CHBrCH2Br����
�ʴ�Ϊ��CH2=CH2+Br2��CH2BrCH2Br����дCH3CH=CH2+Br2��CH3CHBrCH2Br�����ӳɣ�
��4��ʯ���ڴ��������������£����ȿ����ѻ�Ϊ��������̬�������Ͳ�������������Һ̬�ı������Ͳ����������ʴ�Ϊ���ۢܣ�
���� ���⿼���˿�����̽��ʯ���͵ķֽ⼰��ϩ�Ļ�ѧ���ʣ���Ŀ�Ѷ��еȣ�ע�����ճ����л���ṹ�����ʣ���ȷʯ���ͷֽ���P���鷽������������������ѧ�����Ӧ����ѧ֪ʶ��������


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Һ�ܽ����dz������� | |
| B�� | �������ӵ�ֱ����1nm��100nm | |
| C�� | ��������������Ĥ���˷�������Һ�ܽ��еķ�ɢ�����ɢ�� | |
| D�� | �������ö����ЧӦ���ֽ������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na2S2O3�ǻ�ԭ�� | |
| B�� | ���ݸ÷�Ӧ���ж������ԣ�Cl2��SO42-�� | |
| C�� | ����������HCl | |
| D�� | ������Ӧ�У�ÿ����1mol SO42-��ת��2mole- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | v��CO��=1.6 mol/��L•min�� | B�� | v��N02��=0.9 mol/��L•min�� | ||
| C�� | V��N2��=0.25 mol/��L•min�� | D�� | v��CO2��=1.2 mol/��L•min�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ʳ�� | C�� | ���� | D�� | ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��10 mL��Ͳ��ȡ7.13 mL���� | |
| B�� | �ù㷺pH��ֽ���ij��Һ��pHΪ2.3 | |
| C�� | ��������ƽ����25.20 g NaCl | |
| D�� | ��25ml��ʽ�ζ�����ȡ21.70 mLNaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 89.6L | B�� | 44.8L | C�� | 22.4L | D�� | 11.2L |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
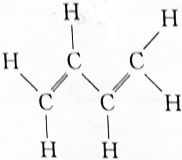 CH2=CH-CH=CH2��=-=��
CH2=CH-CH=CH2��=-=��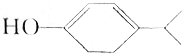 �������ķ���ʽΪC9H14O���������Ϊ�ǻ���̼̼˫����������֬�����������㻯�����֬���������
�������ķ���ʽΪC9H14O���������Ϊ�ǻ���̼̼˫����������֬�����������㻯�����֬����������鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com