
����A�DL 11����������ѧ��ѧ�������ʣ�����C��D��E��FΪ���壬C��D��HΪ���ʡ�1 mol FeC2O4�ڷ�Ӧ����ת��1 mol���ӡ�����������ͼ��ʾ��ת����ϵ�����ַ�Ӧ�е�����������ȥ�����Իش�
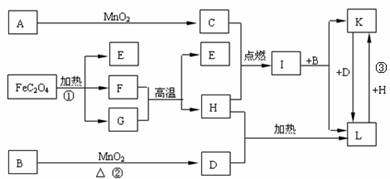
��1��д���й����ʵĻ�ѧʽ��A__________��I____________��
��2��д����Ӧ���л�ѧ����ʽ_____________________________________________��
��3��д����Ӧ���л�ѧ����ʽ_____________________________________________��
��4�� д����Ӧ���е����ӷ���ʽ _____________________________________ ��
 ����������ϵ�д�
����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
(11��)(2011������ģ��)��.�ش������йس��������⣺
(1)Kw����ѧ����ʽ��________���¶�����Kw________(ѡ������С�����䡱)��������pOH����lgc(OH��)����pH��pOH��________(�ú�Kw�Ĵ���ʽ��ʾ)��
(2)Kaͨ����ʾ����ĵ���ƽ�ⳣ����KaֵԽ���ʾ�����������________����ͨ����Ϊ����ȣ�����˼���DZ�ʾ������ʵĵ���̶ȴ�С��һ��ָ�꣬����ijһԪ���ᣬ��������ˮϡ����ʱ��Ka________(ѡ������С�����䡱)����________(ѡ������С�����䡱)��
(3)Ksp��ʾ��������ܶȻ��������ó���Խ���ʾ________��
��.��֪�����£�AgBr��Ksp��4.9��10��13mol2��L��2��AgI��Ksp��8.3��10��17mol2��L��2��
(1)������AgI�ı�����Һ�У�
�ټ������AgNO3����c(I��)________(������С�����䡱����ͬ)��
�����ļӸ����AgI����c(Ag��)________��
�����ļ�AgBr���壬��c(I��)______����c(Ag��)______��
(2)�й������ε��ܶȻ����ܽ��(��AgBr��AgI��)��������������������ȷ����________��
A�����������ε���ʣ�����KspС���ܽ��һ��С
B������AgCl�������Һ�м���������ˮʹAgCl�ܽ��ִﵽƽ��ʱ��AgCl���ܶȻ����䣬���ܽ��Ҳ����
C�������ܵ���ʷ��봿ˮ�У��ܽ�ﵽƽ��ʱ����������ӵ�Ũ�ȵij˻����Ǹ����ʵ��ܶȻ�
D����Һ�д������ֿ�����ͬһ���������ɳ��������ӣ���KspС��һ�������ɳ���
E�������ε���ʵ�Ksp���¶��й�
F��ͬ����ЧӦ(������ԭ����ʾ�����ͬ���ӵ�����)��ʹ�����ε���ʵ��ܽ�ȱ�С��ҲʹKsp��С
(3)������NaBr��KI��Ϊ0.002 mol��L��1����Һ�м���������Ũ��Ϊ4��10��3 mol/L AgNO3��Һ��������ij�����________(�ѧʽ)�����������ټ���������NaI���壬�����տɷ�������ת�����ܷ�Ӧ����ʽ��ʾΪ��______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������������̨���߶���ѧ������������⻯ѧ�Ծ����������� ���ͣ������
��25��ʱ��2L���ܱ�������A��B��C��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±���
| �� �� | A | B | C |
| ��ʼŨ��/mol��L-1 | 1.0 | 2.0 | 0 |
| 2minʱ��ƽ��Ũ��/mol��L-1 | 0.4 | 0.2 | 1.2 |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012��߿���ѧһ�ָ�ϰ����Һ�е����ӷ�Ӧ��ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)(2011������ģ��)��.�ش������йس��������⣺
(1)Kw����ѧ����ʽ��________���¶�����Kw________(ѡ������С�����䡱)��������pOH����lgc(OH��)����pH��pOH��________(�ú�Kw�Ĵ���ʽ��ʾ)��
(2)Kaͨ����ʾ����ĵ���ƽ�ⳣ����KaֵԽ���ʾ�����������________����ͨ����Ϊ����ȣ�����˼���DZ�ʾ������ʵĵ���̶ȴ�С��һ��ָ�꣬����ijһԪ���ᣬ��������ˮϡ����ʱ��Ka________(ѡ������С�����䡱)����________(ѡ������С�����䡱)��
(3)Ksp��ʾ��������ܶȻ��������ó���Խ���ʾ________��
��.��֪�����£�AgBr��Ksp��4.9��10��13mol2��L��2��AgI��Ksp��8.3��10��17mol2��L��2��
(1)������AgI�ı�����Һ�У�
�ټ������AgNO3����c(I��)________(������С�����䡱����ͬ)��
�����ļӸ����AgI����c(Ag��)________��
�����ļ�AgBr���壬��c(I��)______����c(Ag��)______��
(2)�й������ε��ܶȻ����ܽ��(��AgBr��AgI��)��������������������ȷ����________��
| A�����������ε���ʣ�����KspС���ܽ��һ��С |
| B������AgCl�������Һ�м���������ˮʹAgCl�ܽ��ִﵽƽ��ʱ��AgCl���ܶȻ����䣬���ܽ��Ҳ���� |
| C�������ܵ���ʷ��봿ˮ�У��ܽ�ﵽƽ��ʱ����������ӵ�Ũ�ȵij˻����Ǹ����ʵ��ܶȻ� |
| D����Һ�д������ֿ�����ͬһ���������ɳ��������ӣ���KspС��һ�������ɳ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ��߿���ѧһ�ָ�ϰ����Һ�е����ӷ�Ӧ��ר���ۺϲ��ԣ��ս̰棩 ���ͣ�ʵ����
(11��)(2011������ģ��)��.�ش������йس��������⣺
(1)Kw����ѧ����ʽ��________���¶�����Kw________(ѡ������С�����䡱)��������pOH����lgc(OH��)����pH��pOH��________(�ú�Kw�Ĵ���ʽ��ʾ)��
(2)Kaͨ����ʾ����ĵ���ƽ�ⳣ����KaֵԽ���ʾ�����������________����ͨ����Ϊ����ȣ�����˼���DZ�ʾ������ʵĵ���̶ȴ�С��һ��ָ�꣬����ijһԪ���ᣬ��������ˮϡ����ʱ��Ka________(ѡ������С�����䡱)����________(ѡ������С�����䡱)��
(3)Ksp��ʾ��������ܶȻ��������ó���Խ���ʾ________��
��.��֪�����£�AgBr��Ksp��4.9��10��13mol2��L��2��AgI��Ksp��8.3��10��17mol2��L��2��
(1)������AgI�ı�����Һ�У�
�ټ������AgNO3����c(I��)________(������С�����䡱����ͬ)��
�����ļӸ����AgI����c(Ag��)________��
�����ļ�AgBr���壬��c(I��)______����c(Ag��)______��
(2)�й������ε��ܶȻ����ܽ��(��AgBr��AgI��)��������������������ȷ����________��
A�����������ε���ʣ�����KspС���ܽ��һ��С
B������AgCl�������Һ�м���������ˮʹAgCl�ܽ��ִﵽƽ��ʱ��AgCl���ܶȻ����䣬���ܽ��Ҳ����
C�������ܵ���ʷ��봿ˮ�У��ܽ�ﵽƽ��ʱ����������ӵ�Ũ�ȵij˻����Ǹ����ʵ��ܶȻ�
D����Һ�д������ֿ�����ͬһ���������ɳ��������ӣ���KspС��һ�������ɳ���
E�������ε���ʵ�Ksp���¶��й�
F��ͬ����ЧӦ(������ԭ����ʾ�����ͬ���ӵ�����)��ʹ�����ε���ʵ��ܽ�ȱ�С��ҲʹKsp��С
(3)������NaBr��KI��Ϊ0.002 mol��L��1����Һ�м���������Ũ��Ϊ4��10��3 mol/L AgNO3��Һ��������ij�����________(�ѧʽ)�����������ټ���������NaI���壬�����տɷ�������ת�����ܷ�Ӧ����ʽ��ʾΪ��______________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com