
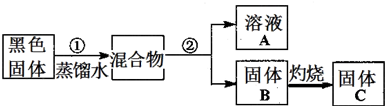
| ʵ����� | Ԥ������ | ���� |
| ����1����ȡ������ҺA��װa��b��c��֧�Թܣ���a�Թܵμ� |
�а�ɫ�������� | ˵����ҺA����Cl- |
| ����2����b�Թܣ� |
˵����ҺA����NH4+ | |
| ����3����c�Թ���μ��� |
�Ȳ��� �� |
˵����ҺA����Zn2+ |
| ||
| ʵ����� | Ԥ������ | ���� |
| ����1����ȡ������ҺA��װa��b��c��֧�Թܣ���a�Թܵμ� ����0.1mol?L-1 AgNO3�� 2mol?L-1 HNO3 |
�а�ɫ�������� | ˵����ҺA����Cl- |
| ����2����b�Թܣ���������6mol?L-1 NaOH��Һ�����ȣ���ʪ��ĺ�ɫʯ����ֽ�����Թܿڸ��� | ʪ��ĺ�ɫʯ����ֽ���� | ˵����ҺA����NH4+ |
| ����3����c�Թ���μ��� ��μ���2mol?L-1 NH3?H2O��6mol?L-1 NaOH������ | �Ȳ��� ��ɫ������ �� �����ܽ� |
˵����ҺA����Zn2+ |
| ||
| ||
| 1 |
| 2 |
| 1 |
| 2 |
| ||
| a |
| 87bv |
| 2��1000a |
| 87bv |
| 2��1000a |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A��һ���¶��£�15g��ȩ������Ļ������Oԭ����Ϊ0.5NA |
| B����״���£�NO��O2��11.2L��ϣ����û������ķ�������Ϊ0.75NA |
| C��14g����ͨʽΪCnH2n�������к��е�C=C����ĿΪNA/n |
| D���ڷ�ӦKIO3+6HI=KI+3I2+3H2O�У�ÿ����3mol I2ת�Ƶĵ�����Ϊ6NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ۢݢߢ� | B���ڢۢߢ� |
| C���ڢݢߢ� | D���ܢڢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
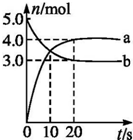 N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��һ���¶��£���2L�̶��ݻ����ܱ������з�����Ӧ2N2O5��g��?4NO2��g��+O2��g����H��0����Ӧ��Ͳ�������������ʵ����淴Ӧʱ��仯��������ͼ��ʾ������˵���У���ȷ���ǣ�������
N2O5��һ�������������������ʺ��Ʊ��ܵ����ǵĹ�ע��һ���¶��£���2L�̶��ݻ����ܱ������з�����Ӧ2N2O5��g��?4NO2��g��+O2��g����H��0����Ӧ��Ͳ�������������ʵ����淴Ӧʱ��仯��������ͼ��ʾ������˵���У���ȷ���ǣ�������| A��0��20 s��ƽ����Ӧ����v��N2O5��=0.1 mol?��L?s��-1 |
| B��10 sʱ�������淴Ӧ������ȣ��ﵽƽ�� |
| C��20 sʱ������Ӧ���ʴ����淴Ӧ���� |
| D������a��ʾNO2�����ʵ����淴Ӧʱ��ı仯 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
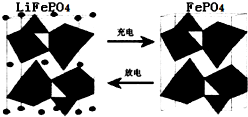
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ����̫���ֽܷ�ˮ����H2���ڴ���������H2��CO2��Ӧ�ϳ�CH3OH����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪��
����̫���ֽܷ�ˮ����H2���ڴ���������H2��CO2��Ӧ�ϳ�CH3OH����������ֱ���Լ״�Ϊȼ�ϵ�ȼ�ϵ�أ���֪��| 1 |
| 2 |
| 3 |
| 2 |
| 1 |
| 2 |
| nB |
| tB |
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
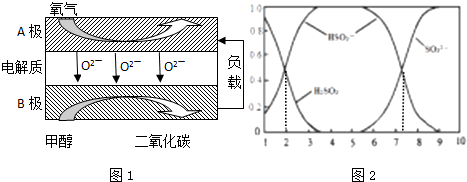
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A���ڻ�ѧ��Ӧ�У��Ͽ���Ӧ���еĻ�ѧ��Ҫ�������� |
| B�����ۻ�����һ�������ۼ������ܺ����Ӽ� |
| C�����ӻ�������ֻ�����Ӽ����������ۼ� |
| D����ѧ��Ӧ�е������仯������Ϊ�����仯 |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com