
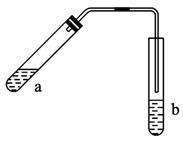
ͼ1-54
(1)�Թ�a����Ҫ����Ũ���ᡢ��������Ҵ���2 mL��
��ȷ�ļ���˳������___________________________________________________��
(2)Ϊ��ֹa�е�Һ����ʵ��ʱ�������У��ڼ���ǰӦ��ȡ�Ĵ�ʩ��____________________��
(3)ʵ���м����Թ�a��Ŀ���ǣ�
��_________________________________________________________________��
��_________________________________________________________________��
(4)�Թ�b�м��б���Na2CO3��Һ����������______________________________________��
(5)��Ӧ���������Թ�b,���ã��۲쵽��������________________________________��
������(1)����Ũ������ϡ�ͻ��ܽ����������ʵĹ����У�������������ȶ����У�����Ӧ��ŨH2SO4���뵽���������С����������������ӷ�����Ϊ������ʧ�����ᡢ�Ҵ������Ȼ���ټ���ŨH2SO4��һ�����˳��Ϊ���ȼ����Ҵ���Ȼ���ҡ�������������H2SO4���ټ�������ᡣ
(2)���Ƕ�Һ����ȵIJ�����Ϊ��ֹҺ�屩�ж�������ʯ��
(3)���Թ�aӦ��С����ȣ�������ȡ�С����ȵ�Ŀ���Ǽӿ컯ѧ��Ӧ���ʣ�������������Ҵ��Ļӷ���������������ƽ���ʱ��������������������������ƽ�����������������ķ����ƶ���
(4)�Թ�b�б���Na2CO3��Һ�������Ҵ��������ᷴӦ�ͽ������������ܽ�ȵ����á�
(5)������������������ˮ�����ܶ�С��ˮ����b�п���������Ϊ��Һ��ֲ㣬�ϲ�Ϊ��ɫ������״Һ�塣
�𰸣�(1)�ȼ����Ҵ���Ȼ���ҡ���Թܱ���������Ũ���ᣬ�ټ��������
(2)���Թ�a�м��뼸����ʯ(�����Ƭ)
(3)�ټӿ췴Ӧ����
�ڼ�ʱ��������������������������ƽ�����������������ķ����ƶ�
(4)�������������������������������ʺ��Ҵ�
(5)b�е�Һ��ֲ㣬�ϲ���������״Һ��


 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�




| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

ͼ1-5-11
��17 g Na2CO3����80 mLˮ�У���װ��C��D�У��ٽ�25 gͭм����Բ����ƿ�У���60 mLŨ�����10 mLˮ�Ļ��Һװ�ڷ�Һ©���У���μ��������С�ļ���ʹSO2����(ע�����������С�ͷ�Ӧ���ʵĿ���)����SO2ͨ��Na2CO3��Һ�������ͣ��ںϲ�C��D��ƿ���õ���Һ�У���������17 g Na2CO3������Ũ������ȴ����������ƾ��塣����ɣ�
(1)�������60 mLŨ������10 mLˮ�Ļ��Һ��
��_________________________________________________________________��
(2)װ��B��Ũ��������ã�____________________________________________________
_____________________________________________________________________��
(3)Na2CO3��ҺҪ��װ��C��D��ƿ�е�������______________��������Ӧ�����ӷ���ʽΪ_____________________________________________________________________��
(4)����SO2�������ʵķ�����___________________________________________________
_____________________________________________________________________��
(5)ͨSO2������Na2CO3��Ŀ����___________________________________________
_____________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
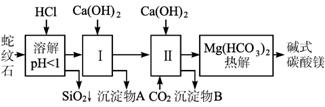
ͼ1-5-21
(1)����ʯ��������ܽ����Һ�����Mg2+�⣬�����еĽ���������__________________.
(2)����1����ʱ��������ҺpH=7-8(�й��������������pH���±�)
�������� | Fe(OH)3 | Al(OH)3 | Mg(OH)2 |
��ʼ����pH | 1.5 | 3.3 | 9.4 |
Ca(OH)2���ܹ�������Ca(OH)2�������ܻᵼ��________�ܽ⡢________������
(3)�ӳ��������A����ȡ��ɫ�����������ϣ����������A�м���________(�������ʵĻ�ѧʽ)��Ȼ��________________________ (������дʵ���������)��
(4)����ѭ��ʹ�ã��ܽ�Լ��Դ������ʵ���У�����ѭ��ʹ�õ�������________________ (��д���ʻ�ѧʽ)��
(5)�����һ��ʵ�飬ȷ����ƷaMgCO3��bMg(OH)2��cH2O��a��b��c��ֵ������������ʵ�鲽��(�����Լ���Ũ���ᡢ��ʯ��)��
����Ʒ���� �ڸ��·ֽ�
��________________________________________________________________
��________________________________________________________________
��MgO����
(6)18.2 g��Ʒ��ȫ�ֽ����6.6 g CO2��8.0 g MgO���ɴ˿�֪����Ʒ�Ļ�ѧʽ�У�
a=________ b=________ c=________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
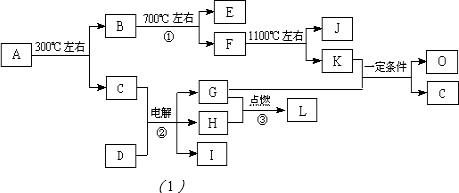
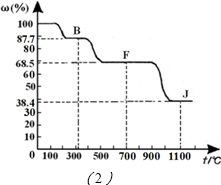
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com