
�����£���0.2 mol��L��1 Al2(SO4)3��Һ�У���μ���1.0 mol��L��1 NaOH��Һ��ʵ������ҺpH��NaOH��Һ����ı仯������ͼ��ʾ�������й�˵����ȷ����(����)
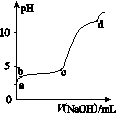
A��a��ʱ����Һ�����Ե�ԭ����Al3��ˮ�⣬���ӷ���ʽΪAl3����3OH�� Al(OH)3
Al(OH)3
B��a��b�Σ���ҺpH����Al3��Ũ�Ȳ���
C��b��c�Σ������OH����Ҫ��������Al(OH)3����
D��d��ʱ��Al(OH)3������ʼ�ܽ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�
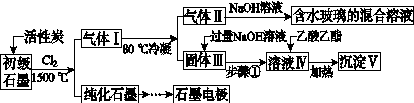
(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�������Ľṹ��ʽΪ �����й��ڱ�������˵����ȷ����(����)
�����й��ڱ�������˵����ȷ����(����)
A���������ɷ�����ȥ��Ӧ���ɱ�Ȳ
B�����������ɱ�ϩ��һ����������ˮ�ӳ��Ƶ�
C����ϩ����ˮ�ӳɺ�����NaOH����Һ����ˮ��ɵñ�����
D����������ͭ�����´������ɵõ�����ȩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������(Na2S2O5)�dz��õ�ʳƷ��������֮һ��ij�о�С���������ʵ�飺
ʵ��һ�����������Ƶ���ȡ
������ͼװ��(ʵ��ǰ�ѳ���װ���ڵĿ���)��ȡNa2S2O5��װ�â�����Na2S2O5���������������ķ�ӦΪNa2SO3��SO2===Na2S2O5��
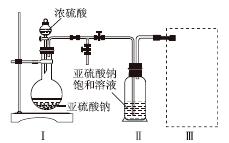
(1)װ�â��в�������Ļ�ѧ����ʽΪ______________________________________��
(2)Ҫ��װ�â��л���������ľ��壬�ɲ�ȡ�ķ��뷽����______��
(3)װ�â����ڴ���β������ѡ�õ������װ��(�г���������ȥ)Ϊ________(�����)��
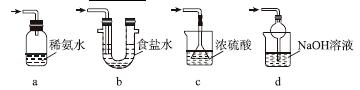
ʵ��������������Ƶ�����
Na2S2O5����ˮ������NaHSO3��
(4)֤��NaHSO3��Һ��HSO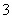 �ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
�ĵ���̶ȴ���ˮ��̶ȣ��ɲ��õ�ʵ�鷽����________(�����)��
a���ⶨ��Һ��pH����b������Ba(OH)2��Һ
c���������� d������Ʒ����Һ
e������ɫʯ����ֽ���
(5)���Na2S2O5�����ڿ������ѱ�������ʵ�鷽����
________________________________________________________________________
________________________________________________________________________��
ʵ���������Ѿ��п��������������IJⶨ
(6)���ѾƳ���Na2S2O5�������������ⶨij���Ѿ��п��������IJ�����(������SO2����)�ķ������£�

(��֪���ζ�ʱ��Ӧ�Ļ�ѧ����ʽΪSO2��I2��2H2O===H2SO4��2HI)
�ٰ���������ʵ�飬���ı�I2��Һ25.00 mL���ô�ʵ������Ʒ�п��������IJ�����(������SO2����)Ϊ________g��L��1��
��������ʵ������У����в���HI��������������ⶨ���________(�ƫ�ߡ���ƫ�͡����䡱)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����£���0.100 mol��L��1 NaOH��Һ�ֱ�ζ�20.00 mL 0.100 mol��L��1������ʹ��ᣬ�ζ�������ͼ��ʾ������˵����ȷ����(����)
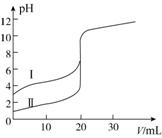
A�����ʾ���ǵζ����������
B��pH��7ʱ���ζ��������ĵ�V(NaOH)С��20 mL
C��V(NaOH)��20.00 mLʱ��������Һ��c(Cl��)��c(CH3COO��)
D��V(NaOH)��10.00 mLʱ��������Һ��c(Na��)>c(CH3COO��)>c(H��)>c(OH��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�о����������������ڴ����к������ӵ������ʱ���漰���·�Ӧ��
2NO2(g)��NaCl(s) NaNO3(s)��ClNO(g)��K1����H1<0(��)
NaNO3(s)��ClNO(g)��K1����H1<0(��)
2NO(g)��Cl2(g) 2ClNO(g)��K2����H2<0(��)
2ClNO(g)��K2����H2<0(��)
(1)4NO2(g)��2NaCl(s) 2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
2NaNO3(s)��2NO(g)��Cl2(g)��ƽ�ⳣ��K��________(��K1��K2��ʾ)��
(2)Ϊ�о���ͬ�����Է�Ӧ(��)��Ӱ�죬�ں��������£���2 L�����ܱ������м���0.2 mol NO��0.1 mol Cl2��10 minʱ��Ӧ(��)�ﵽƽ�⡣���10 min��v(ClNO)��7.5��10��3 mol��L��1��min��1����ƽ���n(Cl2)��________mol��NO��ת������1��________�������������ֲ��䣬��Ӧ(��)�ں�ѹ�����½��У�ƽ��ʱNO��ת���ʦ�2________��1(�>����<������)��ƽ�ⳣ��K2______(�������С�����䡱)����ҪʹK2��С���ɲ�ȡ�Ĵ�ʩ��________��
(3)ʵ���ҿ���NaOH��Һ����NO2����ӦΪ2NO2��2NaOH===NaNO3��NaNO2��H2O����0.2 mol NaOH��ˮ��Һ��0.2 mol NO2ǡ����ȫ��Ӧ��1 L��ҺA����ҺBΪ0.1 mol��L��1��CH3COONa��Һ��������Һ��c(NO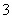 )��c(NO
)��c(NO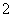 )��c(CH3COO��)�ɴ�С��˳��Ϊ____________________________________________��(��֪HNO2���볣��Ka��7.1��10��4 mol��L��1��CH3COOH�ĵ��볣��Ka��1.7��10��5 mol��L��1)
)��c(CH3COO��)�ɴ�С��˳��Ϊ____________________________________________��(��֪HNO2���볣��Ka��7.1��10��4 mol��L��1��CH3COOH�ĵ��볣��Ka��1.7��10��5 mol��L��1)
��ʹ��ҺA����ҺB��pH��ȵķ�����________��
a������ҺA�м�����ˮ
b������ҺA�м�����NaOH
c������ҺB�м�����ˮ
d������ҺB�м�����NaOH
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������ͼ��ʾװ�ý�������ʵ�飬�ܵó���Ӧʵ����۵���(����)
| ѡ �� | �� | �� | �� | ʵ����� |
|
| A. | ϡ �� �� | Na2S | AgNO3�� AgCl�� ��Һ | Ksp(AgCl)> Ksp(Ag2S) | |
| B. | Ũ �� �� | ���� | ��ˮ | Ũ��������� ˮ�ԡ������� | |
| C. | ϡ �� �� | Na2SO3 | Ba(NO3)2 ��Һ | SO2������� ���ξ������� ��ɫ���� | |
| D. | Ũ �� �� | Na2CO3 | Na2SiO3 ��Һ | ���ԣ�����> ̼��>���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
25 ��ʱ��Ksp(BaSO4)��1��10��10��Ksp(BaCO3)��2.6��10��9�����¶��£�����˵������ȷ����(����)
A����ͬŨ�ȵ�Na2SO4��Na2CO3�Ļ����Һ�еμ�BaCl2��Һ��BaSO4������
B����BaCO3������Һ�м���������������ˮ��c(Ba2��)����
C��BaSO4��BaCO3���������Һ�У�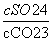 ��
��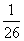
D����BaSO4������Һ�м���Na2CO3��Ũ��Һ��BaSO4������ת��ΪBaCO3
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʵ�鷽������������(����)
A����������ˮ��������������ǣ�ֱ����ˮ��Һ�м�������Cu(OH)2����Һ
B������֯��ɷ�����˿��������˿�������յķ���
C�������Ҵ��������������������̼������Һ
D�������������ϩ������������ֱ�ͨ��������Ȼ�̼��Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com