
 CH3CH2OH+NaCl
CH3CH2OH+NaCl
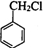 ��
��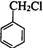 �ڼ���������ˮ������FΪ
�ڼ���������ˮ������FΪ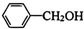 ��F��һ����������CH3COOH����������Ӧ����HΪ
��F��һ����������CH3COOH����������Ӧ����HΪ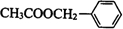 ������л���Ľṹ�����ʽ����⣮
������л���Ľṹ�����ʽ����⣮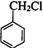 ��
��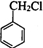 �ڼ���������ˮ������FΪ
�ڼ���������ˮ������FΪ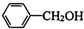 ��F��һ����������CH3COOH����������Ӧ����HΪ
��F��һ����������CH3COOH����������Ӧ����HΪ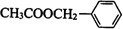 ��
��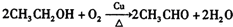 ��
��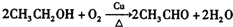 ��
��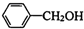 ��һ����������CH3COOH����������Ӧ����
��һ����������CH3COOH����������Ӧ����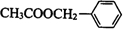 ����Ӧ��ѧ����ʽΪ��
����Ӧ��ѧ����ʽΪ��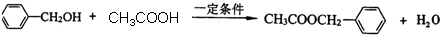 ��
��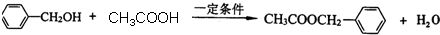 ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| �� |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���㽭ʡ������ѧ2010��2011ѧ���һ��ѧ����ĩ���Ի�ѧ���� ���ͣ�022
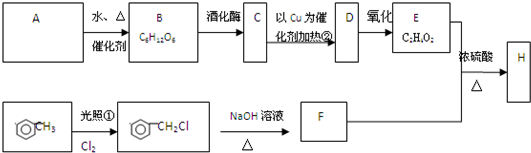
�Ե���AΪԭ�Ͽ����Ƶ���������ζ��H���ְ����·�ʽ���кϳɸ�����(������A��B��C��D��E��F��Ϊ�л��ͬʱ������ijЩ������ʡ��)
��֪
��CH3CH2Cl��NaOH CH3CH2OH��NaCl
CH3CH2OH��NaCl
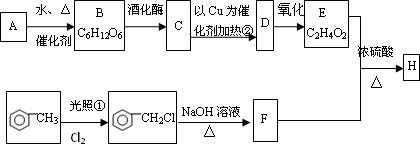
(1)A�Ļ�ѧʽ________��B������________��
(2)��д���٢ڵķ�Ӧ���ͣ���________����________��
(3)C��D�Ļ�ѧ����ʽ________��
(4)F��E��H�Ļ�ѧ����ʽ________��
(5)д��C������ͬ���칹��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꽭��ʡ�����и�һ��ѧ����ĩ��ѧ�������ۻ�ѧ�Ծ����������� ���ͣ������
�Ե���AΪԭ�Ͽ����Ƶ���������ζ��H���ְ����·�ʽ���кϳɸ����ϣ�������A��B��C��D��E��F��Ϊ�л��ͬʱ������ijЩ������ʡ�ԣ�
��֪��RCH2Cl��NaOH RCH2OH��NaCl
RCH2OH��NaCl
��ش��������⣺
��1��A�Ļ�ѧʽΪ______��B������Ϊ______��
��2��д���٢ڵķ�Ӧ���ͣ���______����______��
��3��C��D�Ļ�ѧ����ʽΪ______��
��4��F+E��H�Ļ�ѧ����ʽΪ______��
��5��д��C������ͬ���칹��Ľṹ��ʽ______��______��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�����и�һ��ѧ����ĩ��ѧ�������ۻ�ѧ�Ծ��������棩 ���ͣ��ƶ���
�Ե���AΪԭ�Ͽ����Ƶ���������ζ��H���ְ����·�ʽ���кϳɸ����ϣ�������A��B��C��D��E��F��Ϊ�л��ͬʱ������ijЩ������ʡ�ԣ�
��֪��RCH2Cl��NaOH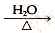 RCH2OH��NaCl
RCH2OH��NaCl

��ش��������⣺
��1��A�Ļ�ѧʽΪ______��B������Ϊ______��
��2��д���٢ڵķ�Ӧ���ͣ���______����______��
��3��C��D�Ļ�ѧ����ʽΪ______��
��4��F+E��H�Ļ�ѧ����ʽΪ______��
��5��д��C������ͬ���칹��Ľṹ��ʽ______��______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com