
���� ��1��100mL 0.2mol/L NaClO��ҺΪǿ�������ε���Һ�������������ˮ����Һ�Լ��ԣ�
��2����Һ��Ϊ100mL pH=1��HNO3��Һ���������Ӻ�ˮ�������������Ũ����ͬ��������ӻ��������㣻
��3������Һ��Ϊ��Һ�ٺ���Һ�ڻ����Һ���õ���Ũ�ȵ�NaNO3��HClO��NaClO������pH=9��˵����Һ�Լ��ԣ������������ˮ��̶ȴ��ڴ�����ĵ���̶ȣ�
����Һ��Ϊ100mL 0.2mol/L NaClO��Һ����Һ��Ϊ100mL pH=1HNO3��Һ����������غ������
����Һ��Ϊ��Һ�ٺ���Һ�ڻ����Һ���õ���Ũ�ȵ�NaNO3��HClO��NaClO������pH=9��˵����Һ�Լ��ԣ������������ˮ��̶ȴ��ڴ�����ĵ���̶ȣ��õ���Һ��Na+��ClO-��NO3-��OH-��H+����Ũ�ȣ�
��� �⣺��1��100mL 0.2mol/L NaClO��ҺΪǿ�������ε���Һ�������������ˮ����Һ�Լ��ԣ���Ӧ�����ӷ���ʽΪ��ClO-+H2O?HClO+OH-��
�ʴ�Ϊ��� ClO-+H2O?HClO+OH-��
��2����Һ��Ϊ100mL pH=1��HNO3��Һ���������Ӻ�ˮ�������������Ũ����ͬ��c��OH-��=c��H+��=$\frac{1{0}^{-14}}{1{0}^{-1}}$mol/L=1��10-13mol/L��
�ʴ�Ϊ��1��10-13��
��3��������Һ��Ϊ��Һ�ٺ���Һ�ڻ����Һ���õ���Ũ�ȵ�NaNO3��HClO��NaClO������pH=9��˵����Һ�Լ��ԣ������������ˮ��̶ȴ��ڴ�����ĵ���̶���Һ��c��ClO-��С��c��NO3-����
�ʴ�Ϊ��С�ڣ�
II����Һ��Ϊ��Һ�ٺ���Һ�ڻ����Һ������pH=9����������غ��������Һ��Ϊ��Һ�ٺ���Һ�ڻ����Һ������pH=9��c��ClO-��+c��HClO��=0.1mol/L��
�ʴ�Ϊ��0.1��
III����Һ��Ϊ��Һ�ٺ���Һ�ڻ����Һ���õ���Ũ�ȵ�NaNO3��HClO��NaClO������pH=9��˵����Һ�Լ��ԣ������������ˮ��̶ȴ��ڴ�����ĵ���̶ȣ��õ���Һ��Na+��ClO-��NO3-��OH-��H+����Ũ�ȴ�СΪ��c��Na+����c��NO3-����c��ClO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��NO3-����c��ClO-����c��OH-����c��H+����
���� ���⿼��������ˮ�⡢������ʵ���ƽ�⡢����Ũ�ȴ�С�ȣ����ջ����ǽ���ؼ�����Ŀ�Ѷ��еȣ�


 �����ÿ�ʱѵ��ϵ�д�
�����ÿ�ʱѵ��ϵ�д� ��Ԫȫ��������ϵ�д�
��Ԫȫ��������ϵ�д� �»ƸԱ����ܾ�ϵ�д�
�»ƸԱ����ܾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ʵ�����Ʊ�����ʱ�ü�Һ����β����Cl2+OH-�TCl-+ClO-+H2O | |
| B�� | ̼�������еμӳ���ʯ��ˮ��CO32-+Ca2+�TCaCO3�� | |
| C�� | ����ʯ�������ܽ⣺CaCO3+2H+�TCa2++CO2��+H2O | |
| D�� | ��ʴ��·���ԭ����Fe3++Cu�TFe2++Cu2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������һ�ְ뵼����ϣ�������������ά | |
| B�� | ���ͷ��к���̼�����ƣ���ʹ���Ƴ��ĸ�����ɶ�� | |
| C�� | ͭ�����Ȼ�����Һ��Ӧ���÷�Ӧ����Ӧ����ӡˢ��·������� | |
| D�� | �����ƿ�����ұ���ѵȽ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
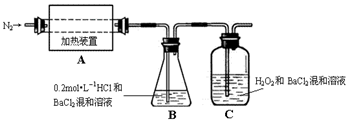
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������177 | B�� | ��������117 | C�� | ���������117 | D�� | ��������177 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ̼����ֱ���������京������̼ԭ������ͬ���������� | |
| B�� | ��Է�������Խ������������ܶȿ���Խ�� | |
| C�� | �л����ڿ����г��ȼ�գ���Ԫ�ص����ղ���ֱ���C��CO2��H��H2O | |
| D�� | �ڿ��Ʒ�Ӧ��Ͳ����Ϊ��̬�������£���������ԭ����Ϊ4������ȼ�շ�Ӧǰ��������� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com