
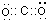 ��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�����ʽΪN2O����ṹʽΪO=N=N��
��һ����B��C��ɵĻ�������AC2��Ϊ�ȵ����壬�����ʽΪN2O����ṹʽΪO=N=N�� ��E�Ļ�̬ԭ�ӵļ����Ų�ʽ��[Ar]3d54s1��ECl3�γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3��
��E�Ļ�̬ԭ�ӵļ����Ų�ʽ��[Ar]3d54s1��ECl3�γɵ������Ļ�ѧʽΪ[Cr��NH3��4��H2O��2]Cl3������ A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�F��Aͬ������A����һ���ڣ�FΪ��Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��� �⣺A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵����A��B��C��D��E������DC�ľ���Ϊ���Ӿ��壬D�Ķ�����������C�������Ӿ�����ͬ�ĵ��Ӳ�ṹ��C�γ�-2�������ӣ���Dλ��C����һ���ڣ�B��C���⻯��ķе������ͬ����������Ԫ���⻯��ķе�ߣ������д��������C�γ�-2�������ӣ���CΪ��Ԫ�أ�DΪþԪ�أ��˵����B��C����BΪ��Ԫ�أ�����A��B��C��ͬһ���ڵķǽ���Ԫ�أ�AC2Ϊ�Ǽ��Է��ӣ���AΪ̼Ԫ�أ�F��Aͬ������A����һ���ڣ�FΪ��Ԫ�أ�E��ԭ������Ϊ24����EΪCrԪ�أ�CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��1��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ�ͬ����������ҵ�һ����������Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�����������ͣ���Ԫ�ص�һ�����ܸ������ڵ�Ԫ�صģ����Ե�һ�������ɴ�С��˳��ΪN��O��C��Mg��
�ʴ�Ϊ��N��O��C��Mg��
��2��AΪ̼Ԫ�أ�BΪ��Ԫ�أ�CΪ��Ԫ�أ�DΪþԪ�أ��ǽ�����Խǿ���縺��Խǿ��ͬ���ڴ����ҵ縺������ǿ����ϡ�������⣩���縺���д�С��˳��ΪO��N��C��Mg��
�ʴ�Ϊ��O��N��C��Mg��
��3��BΪ��Ԫ�أ����⻯��ΪNH3�������к���3��N-H����Nԭ����1�Թ¶Ե��Ӷԣ��ӻ������Ϊ4��Nԭ�Ӳ�ȡsp3�ӻ����۲���ӶԻ���ģ��Ϊ�������壬�ռ乹��Ϊ�����ͣ�
�ʴ�Ϊ���������壻�����ͣ�sp3��
��4��������AC2��CO2��������̼ԭ������ԭ��֮���γ�2�Թ��õ��Ӷԣ�����ʽΪ ��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O���ȵ�����������ƵĽṹ������ṹʽΪ O=N=N��
��һ����NԪ�ء�OԪ�ػ�������CO2��Ϊ�ȵ����壬�仯ѧʽΪN2O���ȵ�����������ƵĽṹ������ṹʽΪ O=N=N��
�ʴ�Ϊ�� ��N2O��O=N=N��
��N2O��O=N=N��
��5��CΪO����̬ԭ�ӵ��������Ų�ͼΪ ��EΪCr��ԭ������Ϊ24��ԭ�Ӻ�����24�����ӣ���̬ԭ�ӵļ����Ų�ʽ��[Ar]3d54s1��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
��EΪCr��ԭ������Ϊ24��ԭ�Ӻ�����24�����ӣ���̬ԭ�ӵļ����Ų�ʽ��[Ar]3d54s1��CrCl3����NH3��H2O�γ�����λ��������������������ʵ���֮��Ϊ2��1������������4��NH3��2��H2O������������λ����磬�������Ϊ[Cr��NH3��4��H2O��2]Cl3��
�ʴ�Ϊ�� ��[Ar]3d54s1��[Cr��NH3��4��H2O��2]Cl3��
��[Ar]3d54s1��[Cr��NH3��4��H2O��2]Cl3��
��6��B������������Ӧ��ˮ����ΪHNO3��D�ĵ���ΪMg��HNO3ϡ��Һ��Mg��Ӧʱ��NԪ�ر���ԭ����ͼۣ�������NH4NO3��Mg������ΪMg��NO3��2����NH4NO3��Mg��NO3��2�Ļ�ѧ�������ֱ�Ϊx��y������ݵ���ת���غ���[5-��-3��]��x=2y������x��y=4��1���÷�Ӧ�Ļ�ѧ����ʽ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
�ʴ�Ϊ��4Mg+10HNO3=4Mg��NO3��2+NH4NO3+3H2O��
��7��AΪC�������۵㼫�ߣ��þ����ǽ��ʯ����Cԭ���γɵ���С������6��Cԭ�ӣ�ÿ��Cԭ���ϵ������������ۼ��ļнǶ���109��28�䣬���ʯΪ��������ṹ��ÿ��̼ԭ����4��̼ԭ��������12g�þ���Ϊ1mol�������Ĺ��ۼ���Ŀ��4��$\frac{1}{2}$��NA=2NA������̼���ɵľ���SiC��ÿ������4��̼֮��4�����ۼ���60g��Ϊ1mol���к���ѧ�������ʵ�����4mol��
�ʴ�Ϊ�����ʯ��6��109��28�䣻2NA��4mol��
��8���ǽ�����Խǿ���⻯����ȶ���Խ�ߣ��⻯���ȶ���˳��ΪH2O��CH4��SiH4������ˮ���Ӽ��γ����ʹ�е��������ߣ�SiH4��CH4�ṹ���ƣ�ǰ����Է��������Ӽ��������е�ߣ��е�����ΪH2O��SiH4��CH4��
�ʴ�Ϊ��H2O��CH4��SiH4��H2O��SiH4��CH4������ˮ���Ӽ��γ����ʹ�е��������ߣ�SiH4��CH4�ṹ���ƣ�ǰ����Է��������Ӽ��������е�ߣ�
���� ��Ŀ�ۺ��Խϴ��漰�ṹ����Խλ�ù�ϵ��Ԫ�������ɡ�����ʽ���������Ų�����������ӻ����ۡ����ӽṹ��������ԭ��Ӧ�ȣ��Ѷ��еȣ������ʽṹ���ۺ�����Ŀ���Ƕ�ѧ���ۺ������Ŀ��飬�⻯��ķе������ͬ����������Ԫ���⻯��ķе�����ƶϵ�ͻ�ƿڣ�


 ��ѧʵ����ϵ�д�
��ѧʵ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ��� | ʵ����� | ʵ��Ŀ�� |
| A | Cl2��Br2�ֱ���H2��Ӧ | �Ƚ��ȡ���ķǽ�����ǿ�� |
| B | ��MgCl2��AlCl3��Һ�зֱ�ͨ��NH3 | �Ƚ�þ����������ǿ�� |
| C | �ⶨ��ͬ���ʵ���Ũ�ȵ�Na2CO3��Na2SO4��Һ��pH | �Ƚ�̼����ķǽ�����ǿ�� |
| D | Fe��Cu�ֱ���ϡ���ᷴӦ | �Ƚ�����ͭ�Ľ�����ǿ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
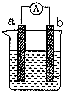 ��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺
��ͼ��ij��ѧ��ȤС��̽����ͬ�����»�ѧ��ת��Ϊ���ܵ�װ�ã���ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ϩ�Ľṹ��ʽ���Ա�ʾΪCH2CH2 | |
| B�� | ��ϩ�ͱ�����ʹ���Ը��������Һ��ɫ | |
| C�� | Һ��ʯ��������Ȼ������Ҫ�ɷֶ��Ǽ��� | |
| D�� | �����Ҵ������ᶼ�ܷ���ȡ����Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 16O��18O | B�� | O2��O3 | C�� | CH3CH2CH3�� | D�� | 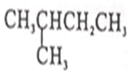 �� �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | P4��NO2���ǹ��ۻ����� | |
| B�� | ��CO2��SiO2�����ж����ڷ��Ӽ������� | |
| C�� | CCl4��NH3�����ж����м��Լ� | |
| D�� | Na2O2�����ӻ����ֻ�������Ӽ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| Ԫ�ش��� | X | W | Y | Z | Q |
| ԭ�Ӱ뾶����10-12m�� | 37 | 64 | 66 | 70 | 154 |
| ��Ҫ���ϼ� | +1 | -1 | -2 | +5��-3 | +1 |
| A�� | Z��X֮���γɵĻ�������л�ԭ�� | |
| B�� | ��Q��Y�γɵĻ�������ֻ�������Ӽ� | |
| C�� | ��X��Y��Z����Ԫ���γɵĻ������ˮ��Һ�ʼ��� | |
| D�� | Y��W�γɵĻ������У�Y�Ը��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | Ŀ�� | ���� |
| A | ȷ��NaCl��Һ���Ƿ����Na2CO3 | ȡ������Һ�μ�CaCl2��Һ���۲��Ƿ���ְ�ɫ���� |
| B | ��ȥKNO3������NaCl | ��������Ƴ��ȵı�����Һ����ȴ�ᾧ������ |
| C | ��ȥ���ᱵ�����е�̼�ᱵ | ��ʢ������������ձ��м���ù��壬�ò��������Ͻ��貢��ֽ��ݣ����ã����ˣ�ϴ�ӣ���� |
| D | ����100mL1.0mol/LCuSO4��Һ | ��25gCuSO4•5H2O����100mL����ˮ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com